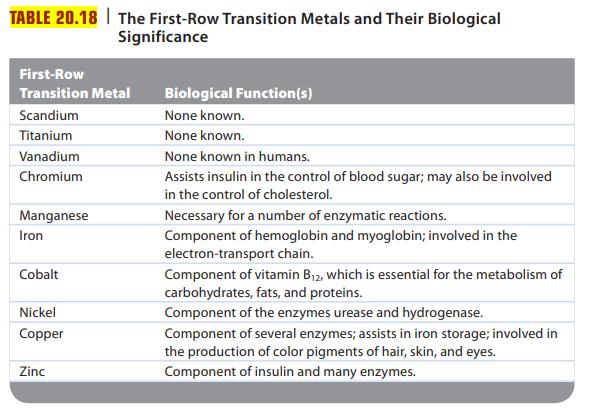
Review Table 20.18, which lists some important biological functions associated with different first-row transition metals. The transport
Question:
Review Table 20.18, which lists some important biological functions associated with different first-row transition metals. The transport of O2 in the blood is carried out by hemoglobin. Briefly explain how hemoglobin transports O2 in the blood.
Transcribed Image Text:
TABLE 20.18 The First-Row Transition Metals and Their Biological Significance First-Row Transition Metal Scandium Titanium Vanadium Chromium Manganese Iron Cobalt Nickel Copper Zinc Biological Function(s) None known. None known. None known in humans. Assists insulin in the control of blood sugar; may also be involved in the control of cholesterol. Necessary for a number of enzymatic reactions. Component of hemoglobin and myoglobin; involved in the electron-transport chain. Component of vitamin B₁2, which is essential for the metabolism of carbohydrates, fats, and proteins. Component of the enzymes urease and hydrogenase. Component of several enzymes; assists in iron storage; involved in the production of color pigments of hair, skin, and eyes. Component of insulin and many enzymes.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
Hemoglobin is a protein found in red blood cells that transports oxygen from the lungs to the tissue...View the full answer

Answered By

Ankit Mahajan
I am an electrical engineering graduate from Thapar institute of engineering and technology.
Qualified exams - GATE 2019,2020.
CAT EXAM 2021- 91.4 percentile
SSC EXAMS- 2019,2020,2021
AFCAT EXAM- 2019,2020,2021
I want to share my knowledge with other people so that they can achieve the same.
I have strong hold Mathematics, Electrical engineering and all the subjects related.
Just give me a problem and I will give you the solution of it.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry An Atoms First Approach
ISBN: 9781305079243
2nd Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl
Question Posted:
Students also viewed these Sciences questions
-
The transport of O 2 in the blood is carried out by hemoglobin. Carbon monoxide (CO) can interfere with O 2 transport because hemoglobin has a stronger affinity for CO than for O 2 . If CO is...
-
Global Touch Corporation (GTC) is one of Canada's largest public companies. GTC provides end users with networking capabilities through its system of copper and coaxial cable lines. GTC operates in...
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
Explain why entrepreneurial firms are often in a strong position to use combination strategies.
-
An inexperienced accountant prepared this condensed income statement for Hight Company, a retail firm that has been in business for a number of years. HIGHT COMPANY Income Statement For the Year...
-
True Or False With both trespass to chattels and conversion, the defendant has a right to return the property to the plaintiff to mitigate their damages.
-
On 1 August 2006 X Ltd granted a 20-year lease to Y Ltd on the following terms: An annual rental of 20,000, payable quarterly in advance on 1 August, 1 November, 1 February and 1 May each year. A...
-
Mrs. Tolstoy and her husband, Serge, are planning their dream house. The lot for the house sits high on a hill with a beautiful view of the Appalachian Mountains. The plans show the size of the house...
-
Pan Corp. is organized into four operating segments. The following segment information was generated by the internal reporting system in 2021: Revenues from Outsiders Intersegment Revenues Operating...
-
a. Determine the magnitude of the resultant force. (Figure 1) b. Determine the coordinate direction angle of the resultant force. c. Determine the coordinate direction angle of the resultant force....
-
Define and give an example of each of the following. a. Roasting b. Smelting c. Flotation d. Leaching e. Gangue What are the advantages and disadvantages of hydrometallurgy? Describe the process by...
-
Why do tetrahedral complex ions have a different crystal field diagram than octahedral complex ions? What is the tetrahedral crystal field diagram? Why are virtually all tetrahedral complex ions high...
-
Recall Exercises 6.2.34 and 6.2.35 about comparing distances traveled by captive African and Asian elephants. a. Put the data in the Multiple Means applet to create a null distribution and from it...
-
# III: Worksheet 3 1. A 20 kg mass is allowed to accelerate down a frictionless 15 ramp. 20 kg 15 a. Draw a force diagram for the block. b. Determine the value of the x-component of the force of...
-
3.Baker Corporation has provided the following production and average cost data for two levels of monthly production volume. The company produces a single product Production Volume: 1,000 units:...
-
Suppose that you own the only company in the market to produce a certain product, and therefore you can determine the market price P dollars for each unit. Due to government regulations, the price of...
-
describes how the blast pressure front can bounce off solid, immovable obstacles and be redirected in another direction in a linear angle to the angle of the obstacle hat was struck
-
As the accounting clerk, you are tasked by the CFO to determine the cost of goods sold of Del Mundo Company for the year ended December 31, 2020. During Operating cost data annd inventory account...
-
Name reasons for the development of wireless ATM. What is one of the main differences to Internet technologies from this point of view? Why did WATM not succeed as stand-alone technology, what parts...
-
A routine activity such as pumping gasoline can be related to many of the concepts studied in this text. Suppose that premium unleaded costs $3.75 per gal. Work Exercises in order. Use the...
-
Photoionization of a diatomic molecule produces a singly charged cation. For the molecules listed here, calculate the bond order of the neutral molecule and the lowest energy cation. For which of the...
-
What would the intensity versus frequency plot in Figure 25.10 look like if fluorescence were fast with respect to internal conversion? Fluorescence Absorption 0-4 Frequency So Absorption...
-
What aspect of the confocal microscope makes single-molecule spectroscopy in solutions possible?
-
whether the following statements is TRUE or FALSE by providing a brief explanation . b) The Value at Risk of a first project is 8 and the Value at Risk of a second project is 5. The Value at Risk of...
-
Fill out a spreadsheet using the following information to perform a NPV analysis. Revenues in each of years 1-3 = $20,000 Year 0 initial investment = $40,000 Inventory level = $10,000 in year 1,...
-
A firm has $9.5 Billion debt outstanding, with a yield to maturity of 5.3% and a coupon rate of 4.6%. They have 148 million preferred shares outstanding, currently trading at $92.29. They also have...

Study smarter with the SolutionInn App


