The Hg 2+ ion forms complex ions with I - as follows: A solution is prepared by
Question:
The Hg2+ ion forms complex ions with I- as follows:
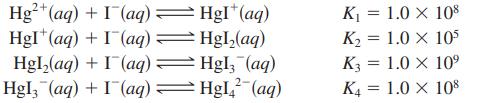
A solution is prepared by dissolving 0.088 mole of Hg(NO3)2 and 5.00 moles of NaI in enough water to make 1.0 L of solution.
![a. Calculate the equilibrium concentration of [HgI4]. b. Calculate the equilibrium concentration of [I]. c.](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1699/9/6/4/82265536796b3c181699964823549.jpg)
Transcribed Image Text:
Hg²+ (aq) + I (aq) = HgI+ (aq) + (aq) = (aq): Hgl₂(aq) + Hgl, (aq) + (aq) → = HgI*(aq) Hgl₂(aq) Hgl, (aq) HgI₂² (aq) K₁ = 1.0 X 108 K₂ = 1.0 X 105 K3 = 1.0 X 109 K4 1.0 X 108
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
The Hg2 ion forms complex ions with I as follows Hg2 I HgI HgI I HgI2 HgI2 I HgI3 HgI3 I HgI4 The eq...View the full answer

Answered By

Nazrin Ziad
I am a post graduate in Zoology with specialization in Entomology.I also have a Bachelor degree in Education.I posess more than 10 years of teaching as well as tutoring experience.I have done a project on histopathological analysis on alcohol treated liver of Albino Mice.
I can deal with every field under Biology from basic to advanced level.I can also guide you for your project works related to biological subjects other than tutoring.You can also seek my help for cracking competitive exams with biology as one of the subjects.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry An Atoms First Approach
ISBN: 9781305079243
2nd Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl
Question Posted:
Students also viewed these Sciences questions
-
A solution is prepared by dissolving table salt, sodium chloride, in water at room temperature. a. Assuming there is no significant change in the volume of water during the preparation of the...
-
QUESTION 1 When propane undergoes complete combustion, the products are carbon dioxide and water.? ? ? ? __ C 3 H 8 (g) + __ O 2 (g) ? __ CO 2 (g) + __ H 2 O(g)What are the respective coefficients...
-
A solution is prepared by dissolving 10.8 g ammonium sulfate in enough water to make 100.0 mL of stock solution. A 10.00-mL sample of this stock solution is added to 50.00 mL of water. Calculate the...
-
Shown here is a list published by Electronics Weekly.com of the top five semiconductor companies in the United States by revenue ($ billions). a. Construct a bar chart to display these data. b....
-
Curtis is an executive on a business trip to the West Coast. He has driven his car on this trip and checks into the Hotel Ritz. The hotel has a guarded underground parking lot. Curtis gives his car...
-
(a) Mercury(II) chloride dissolves in water to give poorly conducting solutions, indicating that the compound is largely nonionized in solution-it dissolves as HgCl2 molecules. Describe the bonding...
-
Which industry had grown the most dramatically? a. computers c. business services b. telecommunications d. both a and b
-
The Shell out Corp. owns a piece of petroleum drilling equipment that costs $100,000 and will be depreciated in 10 years by double declining balance depreciation , with conversion to straight-line...
-
Marigold's Place recorded the following data: Units Unit Cost Date Received Sold On Hand 1/1 Inventory 630 $2.30 1/8 Purchased 1040 1670 3.20 1/12 Sold 1220 450 The weighted average unit cost of the...
-
The carrying amount of ABZ Inc. equity at January 1, 2018 was: Equity CU 1,200,000 Share Capital compromising 120,000 shares of CU 10 par value each Share premium 4,800,000 Share Option Reserve...
-
Calculate the mass of manganese hydroxide present in 1300 mL of a saturated manganese hydroxide solution. For Mn(OH)2, K sp = 2.0 10 -13 .
-
Write equations for the stepwise formation of each of the following complex ions. a. Ni(CN) 4 2- b. V(C2O 4 ) 3 3 -
-
Which of the following is a primary responsibility of the CPMT? a. Conducting a building walk-through during an emergency b. Securing financing so that physical infrastructure can be immediately...
-
Melannie Inc. sold $8,200 worth of merchandise on June 1, 2015 on credit. After inspecting the inventory, the customer determined that 10% of the items were defective and returned them to Melannie...
-
2. (20 marks) A firm wishes to produce a single product at one or more locations so that the total monthly cost is minimized subject to demand being satisfied. At each location there is a fixed...
-
Evaluate your own negotiation way. Do you have one? how you consider having an excellent negotiaiton skill could help any business person to achieve its goals.
-
j. Interest was accrued on the note receivable received on October 17 ($100,000, 90-day, 9% note). Assume 360 days per year. Date Description Dec. 31 Interest Receivable Interest Revenue Debit Credit
-
A Chief Risk Officer (CRO) is interested in understanding how employees can benefit from AI assistants in a way that reduces risk. How do you respond
-
Which features of Canada's labor relations system would you transfer to the United States?
-
When a company has a contract involving multiple performance obligations, how must the company recognize revenue?
-
Distinguish between the following terms applied to a set of functions: orthogonal and normalized, and orthonormal.
-
Why can we conclude that the wave function (x, t) = (x) e i (E / h )t represents a standing wave?
-
What is the usefulness of a complete set of functions?
-
1. Harvey Spectre started a Pearson Hardman on 1 August 2021, a law firm where he will be providing legal services to the companys clients. The following information regarding Pearson Hardman is...
-
On October 20, 2021, a company committed to a plan to sell a division that qualified as a component of the entity according to GAAP regarding discontinued operations and was property classified as...
-
You find that the going rate for a home mortgage with a term of 30 years is 7 % APR. The lending agency says that based on your income, your monthly payment can be at most $ 790 . How much can you...

Study smarter with the SolutionInn App


