In the electrolysis of an aqueous solution of Na 2 SO 4 , what reactions occur at
Question:
In the electrolysis of an aqueous solution of Na2SO4, what reactions occur at the anode and the cathode (assuming standard conditions)?
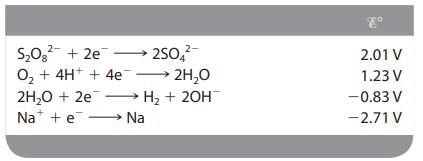
Transcribed Image Text:
2- S₂0² +2e 250 O₂ + 4H+ + 4e →→→ 2H₂O 2H₂O + 2e → H₂ + 2OH Na + e→→→→→→→→→ Na 2.01 V 1.23 V -0.83 V -2.71 V
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
In the electrolysis of an aqu...View the full answer

Answered By

Ann Wangechi
hey, there, paying attention to detail is one of my strong points, i do my very best combined with passion. i enjoy researching since the net is one of my favorite places to be and to learn. i am a proficient and versatile blog, article academic and research writing i possess excellent English writing skills, great proof-reading. i am a good communicator and always provide feedback in real time. i'm experienced in the writing field, competent in computing, essays, accounting and research work and also as a Database and Systems Administrator
4.90+
151+ Reviews
291+ Question Solved
Related Book For 

Chemistry An Atoms First Approach
ISBN: 9781305079243
2nd Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl
Question Posted:
Students also viewed these Sciences questions
-
Write equations for the half-reactions that occur at the anode and cathode in the electrolysis of molten KBr. What are the products formed at the anode and cathode in the electrolysis of aqueous KBr?
-
Which product, O 2 or F 2 , is more likely to form at the anode in the electrolysis of an aqueous solution of KF? Explain your reasoning.
-
Predict the products formed in the electrolysis of an aqueous solution of CdSO 4 .
-
Using Figure 7-5 as an example, redraw Figure 7-12 using an enterprise information system that processes a shared database. Explain the advantages of this system over the paper-based system in Figure...
-
On October 5, Lane Company buys merchandise on account from OBrien Company. The selling price of the goods is $5,000, and the cost to OBrien Company is $3,000. On October 8, Lane returns defective...
-
True Or False Professionals have a fiduciary relationship with their clients if they have given them informed consent.
-
26.1 A Ltd has the following results for the three years to 31 May 2009: y/e 31/5/07 y/e 31/5/08 y/e 31/5/09 Trading profits/(losses) (32,200) 23,800 40,300 Gift Aid donations 400 500 600 Assuming...
-
On January 1, 2010, Alison, Inc., paid $60,000 for a 40 percent interest in Holister Corporations common stock. This investee had assets with a book value of $200,000 and liabilities of $75,000. A...
-
On December 31, 2019, Escapee Company leased machinery from Terminator Corporation for an agreed-upon lease term of 3 years. Escapee agreed to make annual lease payments of $17,000, beginning on...
-
A triage system has been proposed for the ER described in Exercise 3.4. Under the proposed triage plan, entering patients will be registered as before. They will then be quickly examined by a nurse...
-
Gold is produced electrochemically from an aqueous solution of Au(CN) 2 - containing an excess of CN - . Gold metal and oxygen gas are produced at the electrodes. What amount (moles) of O 2 will be...
-
Assign oxidation numbers to all the atoms in each of the following: a. HNO3 b. CuCl c. 0 d. HO e. C6H12O6 f. Ag g. PbSO4 h. PbO i. NaCO4 j. CO k. (NH4)2Ce(SO4)3 1. Cr03
-
Smoking and drinking coffee have a tendency to stain teeth. In an effort to determine the ability of chewing gum to remove stains on teeth, researchers conducted an experiment in which 64 bovine...
-
Question 1 [40 marks] (a) Table 1 present experimental data related to the absorbance of two compounds over a range of concentration, in a UV-Vis cell with path length I = 1.0 cm. From this table:...
-
i. The following table presents data on wholesale gas prices for the major capital cities in the Eastern-half of Australia, from 2011-12 to 2022-23. Use this data to construct a single, time-series...
-
Problem 1 Using the same Fourier-Method approach as used in lecture, consider a beam loaded as shown below. 290 -q. Cos 280 x Shane land V-280 Distributed load w = =-80 . Cos[X] a. What are the...
-
Think about a Floor Warden training program for that company - and write me another email (attached here as a Word document) as if I were the leader of your organization to tell me about the...
-
A particle travels around the curve shown, following ? = ? 0 . 2 ? ? , ?with ? ( ? ) = 0 . 5 ? 2 rad. At the moment ? = ? , ?determine the speed and acceleration of the particle. ? = , ? ? ? = , ? ?...
-
Describe the functions of the MS and SIM. Why does GSM separate the MS and SIM? How and where is user-related data represented/stored in the GSM system? How is user data protected from unauthorized...
-
Separate variables and use partial fractions to solve the initial value problems in Problems 18. Use either the exact solution or a computer-generated slope field to sketch the graphs of several...
-
A surface for which the electrostatic potential is negative delineates regions in a molecule that are subject to electrophilic attack. It can help you to rationalize the widely different chemistry of...
-
Hydrocarbons are generally considered to be nonpolar or weakly polar at best, characterized by dipole moments that are typically only a few tenths of a debye. For comparison, dipole moments for...
-
Chemists know that nitric and sulfuric acids are strong acids and that acetic acid is a weak acid. They would also agree that ethanol is at best a very weak acid. Acid strength is given directly by...
-
The Two Dollar Store has a cost of equity of 10.9 percent, the YTM on the company's bonds is 5.6 percent, and the tax rate is 40 percent. If the company's debtequity ratio is .45, what is the...
-
Which of the following groups have assets under management that are close in dollar value (within $2 trililon) to those of the banking system? Which are larger? Which are smaller? Cite data to...
-
Financial data for Joel de Paris, Inc., for last year follow: Joel de Paris, Inc. Balance Sheet Beginning Balance Ending Balance Assets Cash Accounts receivable Inventory Plant and equipment, net...

Study smarter with the SolutionInn App


