For each of the following aqueous reactions, identify the acid, the base, the conjugate base, and the
Question:
For each of the following aqueous reactions, identify the acid, the base, the conjugate base, and the conjugate acid.
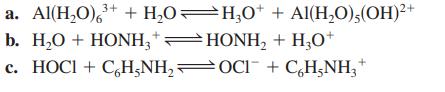
Transcribed Image Text:
3+ a. Al(H₂O) ³+ + H₂O—H₂0* + Al(H₂O),(OH)²+ b. H₂O + HONH3 + HONH₂ + H₂O+ c. HOCI + C₂H5NH₂OCl¯ + C₂H₂NH₂+
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 40% (5 reviews)
Answers Equation Acid Base Conjugate Base Conjugate Acid a AlH2O63 H2O AlH2O...View the full answer

Answered By

Ashington Waweru
I am a lecturer, research writer and also a qualified financial analyst and accountant. I am qualified and articulate in many disciplines including English, Accounting, Finance, Quantitative spreadsheet analysis, Economics, and Statistics. I am an expert with sixteen years of experience in online industry-related work. I have a master's in business administration and a bachelor’s degree in education, accounting, and economics options.
I am a writer and proofreading expert with sixteen years of experience in online writing, proofreading, and text editing. I have vast knowledge and experience in writing techniques and styles such as APA, ASA, MLA, Chicago, Turabian, IEEE, and many others.
I am also an online blogger and research writer with sixteen years of writing and proofreading articles and reports. I have written many scripts and articles for blogs, and I also specialize in search engine
I have sixteen years of experience in Excel data entry, Excel data analysis, R-studio quantitative analysis, SPSS quantitative analysis, research writing, and proofreading articles and reports. I will deliver the highest quality online and offline Excel, R, SPSS, and other spreadsheet solutions within your operational deadlines. I have also compiled many original Excel quantitative and text spreadsheets which solve client’s problems in my research writing career.
I have extensive enterprise resource planning accounting, financial modeling, financial reporting, and company analysis: customer relationship management, enterprise resource planning, financial accounting projects, and corporate finance.
I am articulate in psychology, engineering, nursing, counseling, project management, accounting, finance, quantitative spreadsheet analysis, statistical and economic analysis, among many other industry fields and academic disciplines. I work to solve problems and provide accurate and credible solutions and research reports in all industries in the global economy.
I have taught and conducted masters and Ph.D. thesis research for specialists in Quantitative finance, Financial Accounting, Actuarial science, Macroeconomics, Microeconomics, Risk Management, Managerial Economics, Engineering Economics, Financial economics, Taxation and many other disciplines including water engineering, psychology, e-commerce, mechanical engineering, leadership and many others.
I have developed many courses on online websites like Teachable and Thinkific. I also developed an accounting reporting automation software project for Utafiti sacco located at ILRI Uthiru Kenya when I was working there in year 2001.
I am a mature, self-motivated worker who delivers high-quality, on-time reports which solve client’s problems accurately.
I have written many academic and professional industry research papers and tutored many clients from college to university undergraduate, master's and Ph.D. students, and corporate professionals. I anticipate your hiring me.
I know I will deliver the highest quality work you will find anywhere to award me your project work. Please note that I am looking for a long-term work relationship with you. I look forward to you delivering the best service to you.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry An Atoms First Approach
ISBN: 9781305079243
2nd Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl
Question Posted:
Students also viewed these Sciences questions
-
The conjugate base of diethyl malonate can serve as a nucleophile to attack a wide range of electrophiles. Identify the product that is formed when the conjugate base of diethyl malonate reacts with...
-
Weak base B has a pK b of 6.78 and weak acid HA has a pK a of 5.12. a. Which is the stronger base, B or A ? b. Which is the stronger acid, HA or BH + ? c. Consider the following reaction: B(aq) +...
-
Each of the following reactions has been reported in the chemical literature and gives a single organic product in high yield. Write the structure of the product for each reaction. (a)...
-
A trader depreciates his vehicles at 40% per annum using the reducing balance method, with proportionate depreciation in the year of acquisition. In addition to vehicles acquired on 1 July 2010 for...
-
Rohan, an eighty-three-year-old invalid, employs a nurse, Sarah, to care for him. Prior to Sarahs employment, Rohan executed a will leaving his entire estate to his only living relativehis...
-
Use thermochemical data (Appendix C) to decide whether the equilibrium constant for the following reaction will increase or decrease with temperature. CH4(g) + 2H,S(g) CS2(g) + 4H2(g)
-
Discuss the possible link between personal traits and leadership ability.
-
For the year ended December 31, 2012, the job cost sheets of Dosey Company contained the following data. Other data: 1. Raw materials inventory totaled $20,000 on January 1. During the year, $100,000...
-
points On January 1 Poros Corporation had a beginning balance in retained mornings of $72.000, Dungyear, the corporation has not income of $48.000 and paid out cash vidonda at $20,000. The balance in...
-
For the partially submerged backfill in Problem 13.13 (Figure 13.37), determine the Rankine's passive force per unit length of the wall and the location of the resultant In problem 13 Problem HH Y1 ...
-
The pH of a sample of gastric juice in a persons stomach is 2.1. Calculate the pOH, [H + ], and [OH - ] for this sample. Is gastric juice acidic or basic?
-
How many significant figures are there in the following numbers: 10.78, 6.78, 0.78? If these were pH values, to how many significant figures can you express the [H + ]? Explain any discrepancies...
-
In the current year Alice reports $150,000 of salary income, $20,000 of income from activity X, and $35,000 and $15,000 losses from activities Y and Z, respectively. All three activities are passive...
-
how could playing in a sandbox help to the development of children? how could a garden help to the development of children? how could playground obstacle courses like a pebble bridge and monkey bars...
-
A store order bottles of shampoo throughout the year. Over time, the store has learned that the annual demand D for shampoo is constant, i.e., there is no variability. Currently, the store decides to...
-
Solve the Practice #2 == where L2 =02A = a, L404B = c, L = 0204 = d, y = /2 1) Find the velocity 3 when 82 = /2 and 6 = 0.4 rad/s 2) Find the acceleration 63 when = /2 and 62 = 0.4 rad/s 03. 03 Y B...
-
.0.5 0.5 For the above plot of the ellipsoid (22) 2- + +() + (-) = 1, find the parameters a, b and c. Note that a, b and c are positive integers between 1 and 6 inclusive. Use the mouse to rotate the...
-
The annual energy consumption of the University of Maryland is 100 million kWh. How much Uranium-235 is needed to produce this amount of energy in a nuclear power plant assuming 100% efficiency? (The...
-
What are some similarities and differences between mediation, fact finding, and interest arbitration?
-
Which of the ocean zones shown would be home to each of the following organisms: lobster, coral, mussel, porpoise, and dragonfish? For those organisms you identify as living in the pelagic...
-
For each of the following pairs of compounds, identify which compound is the stronger base: (a) (b) (c) (d) N- N- N- N-
-
Rank the following compounds in terms of increasing basicity: N. Br z-
-
When (E)-4-amino-3-buten-2-one is treated with molecular hydrogen in the presence of platinum, the resulting amine is more basic than the reactant. Draw the reactant and the product, and explain why...
-
06:27 82% . . 4 Document (1) Question Management accounting has evolved over the years to provide information for: determining the cost of product and services and financial control; management...
-
R paid the following state and local taxes during the current year: State income taxes due with his prior year tax return $ 700 State income taxes withheld by his employer 2,500 Real estate taxes...
-
View Policies Current Attempt in Progress Blossom Company owns equipment that cost $ 62, 100 when purchased on January 1, 2019. It has been depreciated using the straight- line method based on...

Study smarter with the SolutionInn App


