Calculate the pH of the following solutions: a. 1.2 M CaBr b. 0.84 M C6H5NH3NO3 (K for
Question:
Calculate the pH of the following solutions:
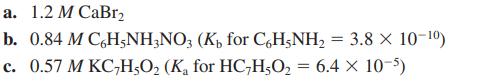
Transcribed Image Text:
a. 1.2 M CaBr₂ b. 0.84 M C6H5NH3NO3 (K₁ for C6H5NH₂ = 3.8 × 10-¹⁰) c. 0.57 M KC₂H5O₂ (K₁ for HC-H₂O₂ = 6.4 x 10-5)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
Solution 1 12 M CaBr CaBr is a salt of a strong base CaOH and a strong acid HBr When dissolved in wa...View the full answer

Answered By

Arun kumar
made more than four thousand assignments
5.00+
3+ Reviews
10+ Question Solved
Related Book For 

Chemistry An Atoms First Approach
ISBN: 9781305079243
2nd Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl
Question Posted:
Students also viewed these Sciences questions
-
Calculate the pH of each of the following solutions (Ka and Kb values are given in Appendix D): (a) 0.095 M propionic acid (C2H5COOH), (b) 0.100 M hydrogen chromate ion (HCrO4-, (c) 0.120 M pyridine...
-
First, write the code to create a single DataFrame object in a function called load_ticket_data(). This function should return the full dataframe and take no parameters (you can assume the ticket...
-
Pioneer Bicycle Shop is an authorized Trek dealer. In order to be an authorized Trek dealer, Pioneer and Trek signed an agreement where Trek agreed to sell Pioneer its entire line of bicycles and...
-
What are the types of production systems in aquaculture?
-
On what did the court base its reasoning for its ruling on this issue?
-
Cyclopropane, C3H6, is converted to its isomer propylene, CH2PCHCH3, when heated. The rate law is first order in cyclopropane, and the rate constant is 6.0 104/s at 500oC. If the initial...
-
You are a supervisor in the engineering department and a member of a team that includes people from production, finance, marketing, and engineering. After conducting marketing research, your team...
-
Fresh Fruits Corporation wholesales peaches and oranges. Lashanda King is working with the company's accountant to prepare next year's budget. Ms. King estimates that sales will increase 5 percent...
-
ener question will save this response. Question 14 2 points On January 1, Madison Co, negotiated a 90 days forward contract to buy 100 milion Japanese yen at Sen 0.009. After 30 days. Madison Co....
-
The 2021 income statement for Bjorgvin Salmon is presented below. Revenue (115,000 Kgs) Expenses Fish Preparation Materials Packaging Materials Direct Labor Administration Commissions Total expenses...
-
Students are often surprised to learn that organic acids, such as acetic acid, contain OOH groups. Actually, all oxyacids contain hydroxyl groups. Sulfuric acid, usually written as H 2 SO 4 , has the...
-
Calculate the pH of each of the following solutions. a. 0.12 M KNO 2 b. 0.45 M NaOCl c. 0.40 M NH 4 ClO 4
-
Rationalize each denominator. Assume that all radicands represent positive real numbers. 4/8
-
Do you think digital wallets will revolutionize electronic banking and in-store transactions? 2. How do you think digital wallets will affect traditional banks? 3. What are some of the risks of...
-
5.14 Strains are measured on the surface of a brass alloy part as follows: Ex 160010-6 y=1300106, and Yxy = 1500106. Estimate the in-plane stresses x, y, and Txy, and also the strain normal to the...
-
E) prepare preclosing trial balances at december 31,2026. for the debt service fund, considering only the proceeds, expenditures, and transfers resulting from transactions of the capital projects...
-
Explain at least 8 types of Google ads brieflyAnalyze the ad & share your opinion on its performance and suggest changes if required. * add the snapshots, and pictures of examples
-
Categorize each variable as quantitative or qualitative GPA is continuous Number of students is Discrete GPA ( Continuous) and Number of Students ( Discrete) GPA ( Discrete) and the Number of...
-
Discuss two reasons grievances might be filed, furnishing examples of these reasons other than those found in the text?
-
How can NAFTA be beneficial to suppliers of Walmart?
-
Nitriles undergo alkylation at the α position much like ketones undergo alkylation at the α position. The α position of the nitrile is first deprotonated to...
-
Identify the Michael donor and Michael acceptor that could be used to prepare each of the following compounds via a Michael addition. (a) (b) (c) (d) (e) OEt N
-
The conjugate base of diethyl malonate can serve as a nucleophile to attack a wide range of electrophiles. Identify the product that is formed when the conjugate base of diethyl malonate reacts with...
-
10. Accounting firm XYZ provides audit tax and general accounting services, it estimates it will have 773 auditing 500 taxation, and 1,000 other service engagements. The company uses an ABC system...
-
Chapter 3: Adjusting Entries for Supplies Account and Unearned Revenue Account (Fee earned meaning Revenue) Q3-1. Western Company had $500 of store supplies available at the beginning of the current...
-
Please show me the table for production budget too, showing all calculations. Thanks Rucker Company manufactures drinking glasses. One unit is a package of eight glasses, which sells for $18. Rucker...

Study smarter with the SolutionInn App


