Order the following solids (ad) from least soluble to most soluble. Ignore any potential reactions of the
Question:
Order the following solids (a–d) from least soluble to most soluble. Ignore any potential reactions of the ions with water.
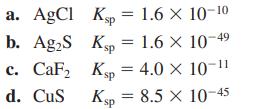
Transcribed Image Text:
a. AgCl Kp = 1.6 × 10-10 Ksp = 1.6 × 10- b. Ag₂S c. CaF₂ d. CuS Ksp = 4.0 × 10-¹¹ Ksp = 8.5 X 10-45
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)
Note When a salt has a larger Ksp value its solubility will be hi...View the full answer

Answered By

David Muchemi
I am a professional academic writer with considerable experience in writing business and economic related papers. I have been writing for my clients who reach out to me personally after being recommended to me by satisfied clients.
I have the English language prowess, no grammatical and spelling errors can be found in my work. I double-check for such mistakes before submitting my papers.
I deliver finished work within the stipulated time and without fail. I am a good researcher on any topic especially those perceived to be tough.
I am ready to work on your papers and ensure you receive the highest quality you are looking for. Please hire me to offer my readily available quality service.
Best regards,
4.60+
27+ Reviews
61+ Question Solved
Related Book For 

Chemistry An Atoms First Approach
ISBN: 9781305079243
2nd Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl
Question Posted:
Students also viewed these Sciences questions
-
Assuming that the solubility of Ca 3 (PO 4 ) 2 (s) is 1.6 10 -7 mol/L at 25 C, calculate the K sp for this salt. Ignore any potential reactions of the ions with water.
-
When a pure substance is placed in contact with water, there are three possible outcomes. The substance may do nothing that is, the substance does not dissolve and no visible change takes place. The...
-
Part 1 a. Ammonia, NH 3 , is a weak electrolyte. It forms ions in solution by reacting with water molecules to form the ammonium ion and hydroxide ion. Write the balanced chemical reaction for this...
-
Perform the indicated operations and simplify the result. Leave your answer in factored form. X x - 3 x + 1 x2 + 5x 24
-
What type of bailment relationship was created when Denai agreed to store Finneys boat? What degree of care was Denai required to exercise in storing the boat? Vanessa Denai purchased forty acres of...
-
Sodium peroxide, Na2O2, consists of Na+ and O22 (peroxide) ions. Write the molecular orbital configuration and bond order of the peroxide ion, O22.
-
How many of the CEOs were graduates of four-year colleges? a. about a fourth c. about three fourths b. nearly half d. all of them
-
Calculate the cash dividends required to be paid for each of the following preferred stock issues: Required: a. The semiannual dividend on 6% cumulative preferred, $50 par value, 30,000 shares...
-
Soto Industries Inc. is an athletic footware company that began operations on January 1, 20Y3. The following are bond (held-to-maturity) transactions by Soto Industries Inc., which has a fiscal year...
-
What specifically, is direct response advertising? What makes it unique from all other types advertising?
-
A 50.0-mL sample of 0.00200 M AgNO 3 is added to 50.0 mL of 0.0100 M NaIO 3 . What is the equilibrium concentration of Ag + in solution? (K sp for AgIO 3 is 3.0 10 -8 .)
-
Calculate the solubility of Co(OH) 2 (s) (K sp = 2.5 10 -16 ) in a buffered solution with a pH of 11.00.
-
Explain two ways to detect an error in arithmetic in bases other than base 10.
-
Business meeting simulation: Start with preparing an email and send it to all the participant of the meeting to inform about the meeting topic/ agenda/time of the meeting/ room #. Create a meeting...
-
Topic #1: Rayleigh-Ritz Method (RRM) Problem 1 a) Find the exact solution for the beam torsion problem shown above. The loading consists of a uniformly distributed torque m and a point torque M...
-
Assume that a $10,000, five-year, 8% term note, is issued on October 1, 20X3: what is the Journal Entry Cash Note Payable 10,000 10,000 Cash 10,000 Accounts Payable 10,000 Note Payable 10,000 Cash...
-
How do I get help with combining a final paper over a period of 6 weeks for my Senior Management Seminar course MGMT-495 at American Public University.
-
Question: Fillmore, Inc. specializes in customized optimization spreadsheet software. The results of the company's operations during the prior year ( 2 0 ?are given in the following table. All...
-
Western Europe seems to be uniquely involved with various forms of worker participation. What are some reasons that these worker participation systems have developed so fully there instead of...
-
In what ways does a well-designed enterprise search software vary from popular search engines (e.g., Bing, DuckDuckGo, and Google)?
-
Identify the reagents that you would use to accomplish each of the following transformations: (a) (b) H.
-
Predict the major product for each of the following transformations: (a)
-
Identify the reagents that you would use to accomplish each of the following transformations (you will also need to use reactions from previous chapters). (a) (b) (c) Br Br OH
-
E6-5 Inferring Missing Amounts Based on Income Statement Relationships [LO 6-2, LO 6-6] Supply the missing dollar amounts for each of the following independent cases: E6-5 Inferring Missing Amounts...
-
The transactions of Spade Company appear below. a. K. Spade, owner, invested $12,250 cash in the company in exchange for common stock. b. The company purchased supplies for $355 cash. c. The company...
-
Urgent Help Needed!!!! The Blatz Furniture Company uses an online data input system for processing its sales invoice data, salesperson data, inventory control, and purchase order data. Representative...

Study smarter with the SolutionInn App


