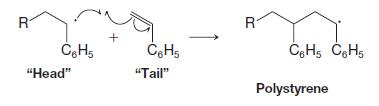
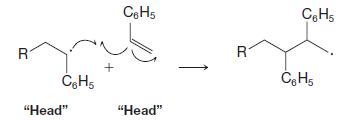
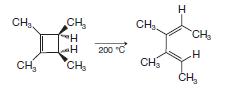
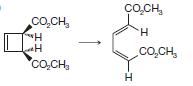
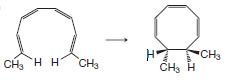
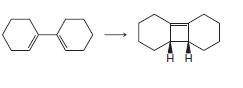
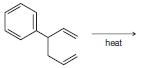
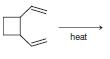
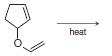
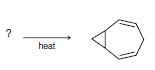
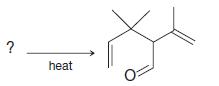
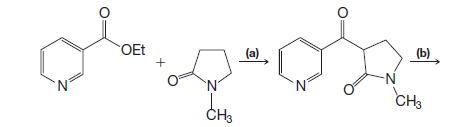
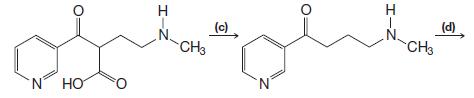
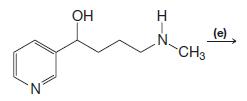
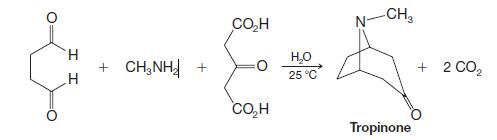
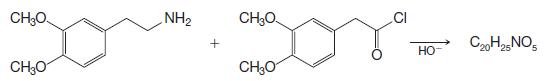
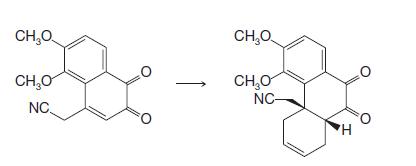
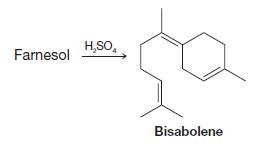
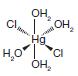
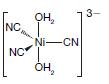
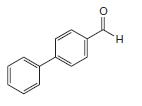
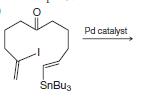
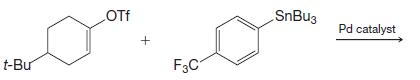
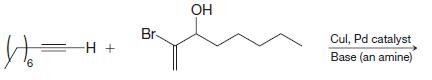
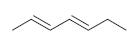
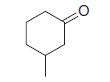
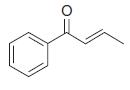
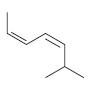
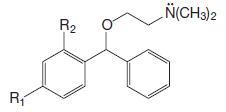
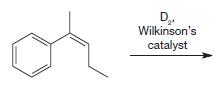
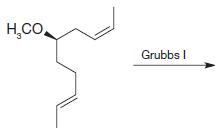
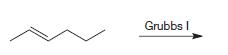
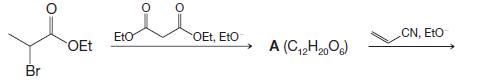
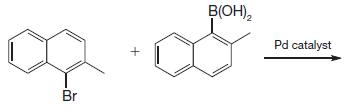
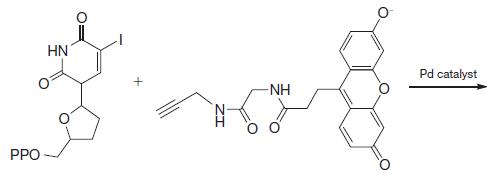
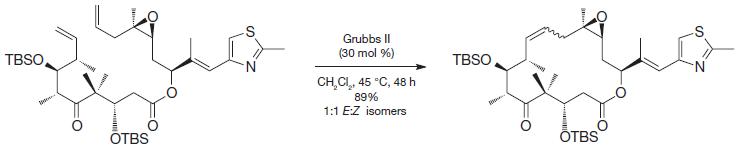
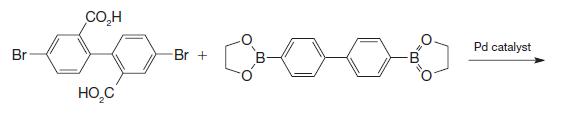
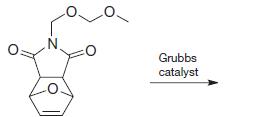
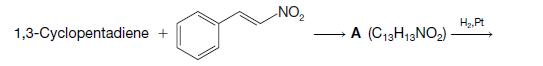
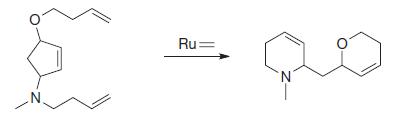
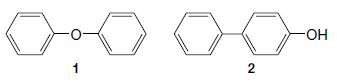
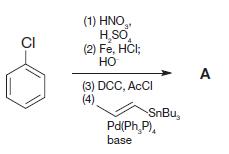
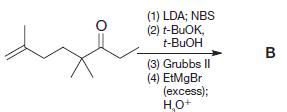
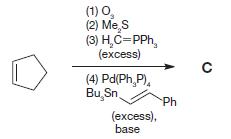
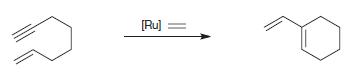
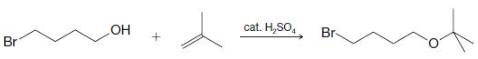
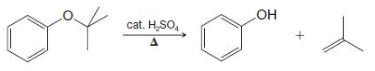
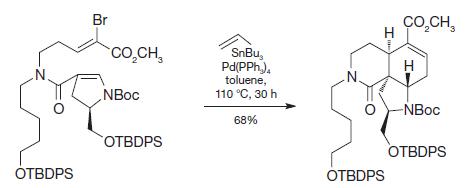
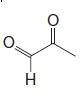
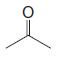
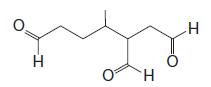
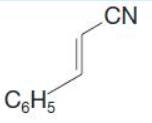
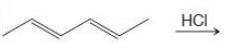
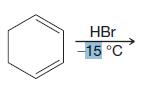
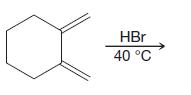
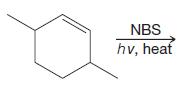
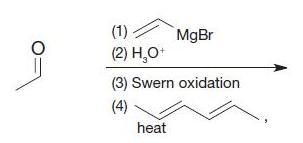
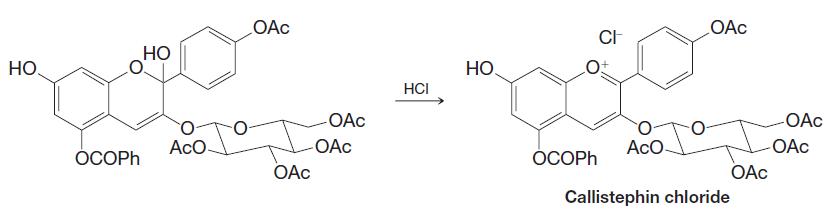
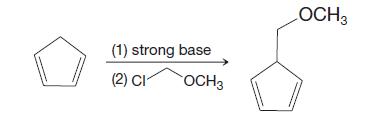
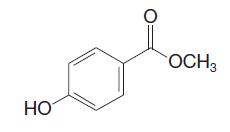
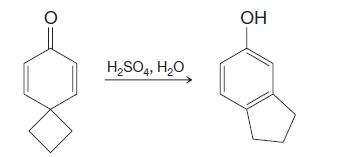
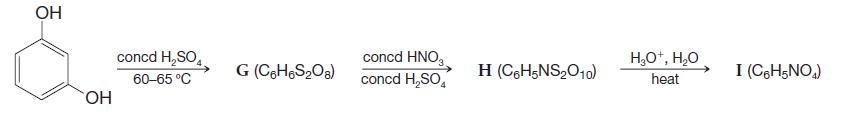
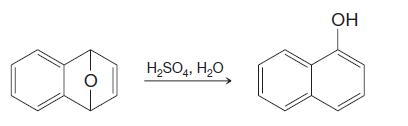
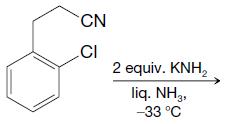
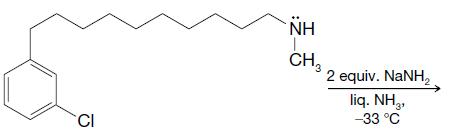
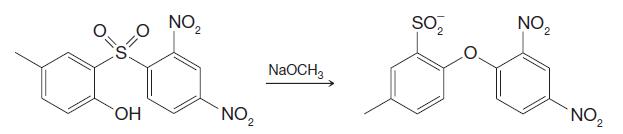
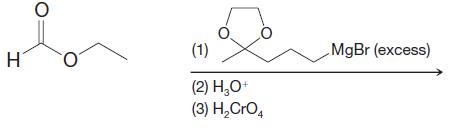
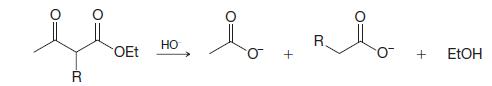
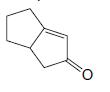
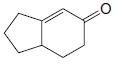
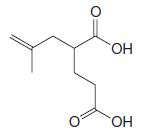
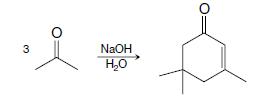
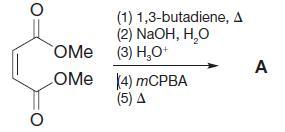
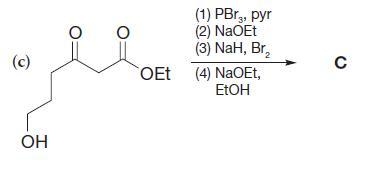
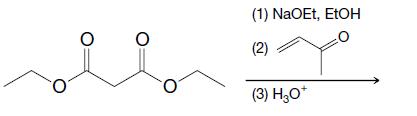
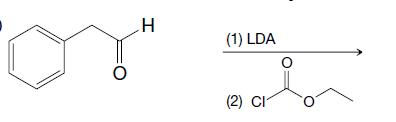
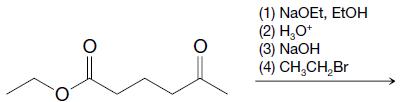
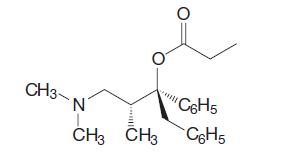
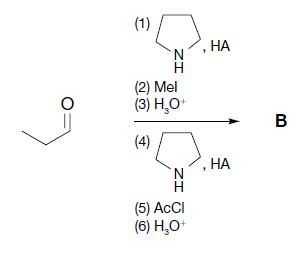
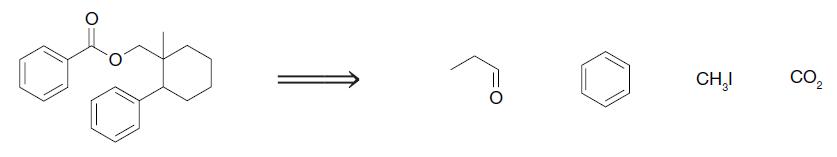
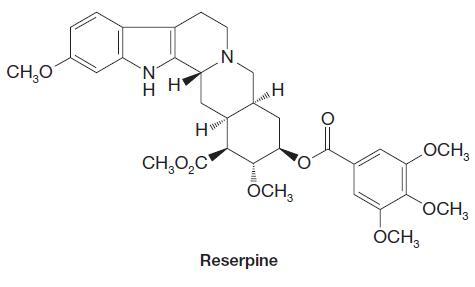
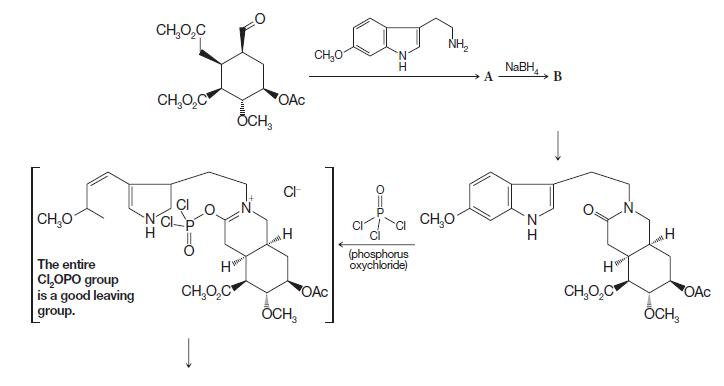
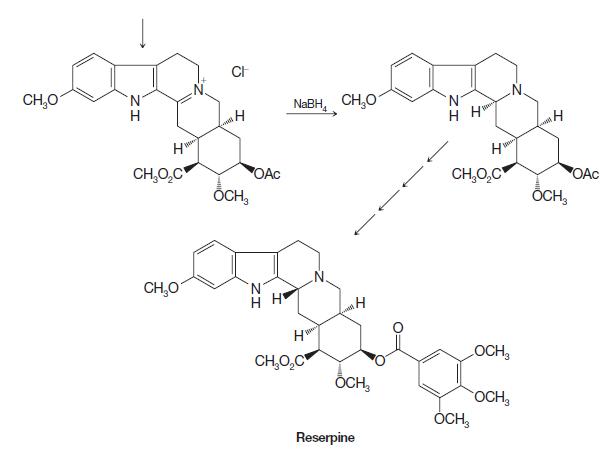
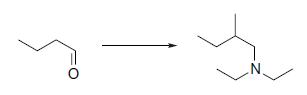
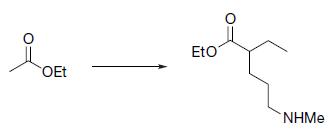
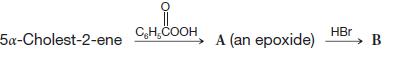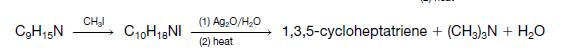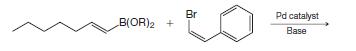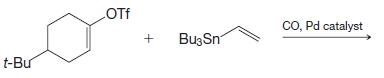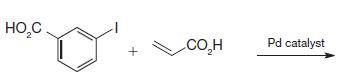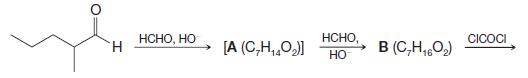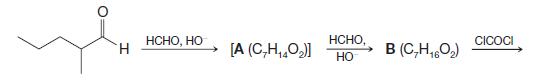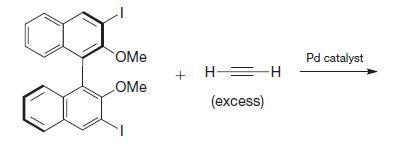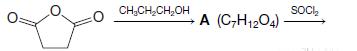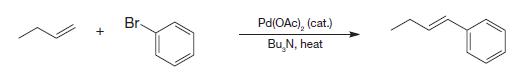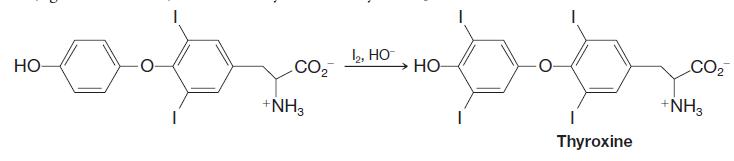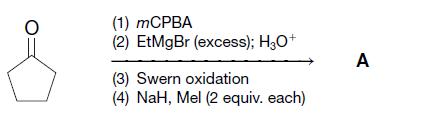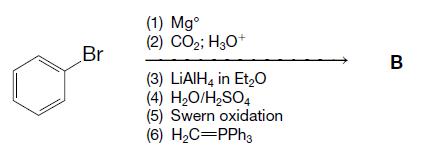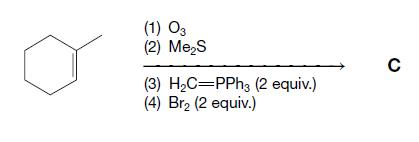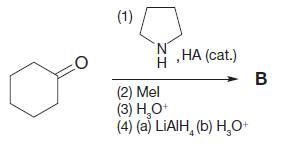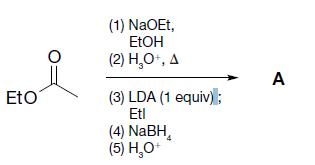Organic Chemistry 12th Edition T. W. Graham Solomons, Craig B. Fryhle, Scott A. Snyder - Solutions
Discover a comprehensive collection of solutions for "Organic Chemistry 12th Edition" by T. W. Graham Solomons, Craig B. Fryhle, and Scott A. Snyder. Access an extensive range of solved problems, chapter solutions, and step-by-step answers to enhance your understanding. Whether you're searching for an online answers key, a solution manual, or a test bank, we offer all resources for free download. Our instructor manual provides in-depth explanations, ensuring you grasp every concept with ease. Dive into our textbook's questions and answers to excel in your studies with the solutions PDF.
![]()
![]() New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
![]()
![]()