Provide a mechanism for the following reaction. H2SO4, H2O OH
Question:
Provide a mechanism for the following reaction.
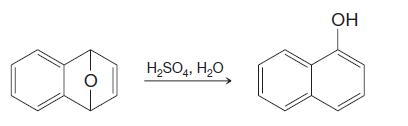
Transcribed Image Text:
H2SO4, H2O OH
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 76% (13 reviews)
Answer The reaction shown can be achieved using a Heck reaction In a Heck reac...View the full answer

Answered By

Justin Akengo
I am writing in application for the tutor position with your organisation. I am experienced in tutoring students of all abilities and I believe I am the ideal candidate for this position.
I work with students of all ages, from elementary school to college level. Whether the subject is science, Mathematics or basic study skills, I break material down into easy-to-understand concepts. In your job posting, you asked for someone who can tutor in a variety of subjects. I am comfortable explaining calculus to a college student or working with a kindergartener on spelling fundamentals.
Below are just a few core skills and qualifications I posses as a tutor;
Adept at creating study materials in a variety of academic subjects to help students improve their test scores and GPAs.
Strong interpersonal skills in working with students to help them achieve and succeed.
Have written study books adopted by a high school and a college to help students improve their skills in English and mathematics.
Have won several “Tutor of the Year” awards for work with high school and college students.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Organic Chemistry
ISBN: 978-1118875766
12th Edition
Authors: T. W. Graham Solomons, Craig B. Fryhle, Scott A. Snyder
Question Posted:
Students also viewed these Sciences questions
-
Provide a mechanism for the following reaction and explain why it occurs faster than nitration of benzene. NO2
-
Provide a mechanism for the following reaction OH H2SO4 H20
-
Provide a mechanism for the following reaction. Draw a reaction energy coordinate diagram that illustrates the kinetic and thermodynamic pathways for this reaction. HBr Br+
-
The membrane filter technique is used to test a polluted water sample for coliform group. Three different dilutions of the water sample were prepared and each was filtered through 5 filter membranes....
-
X + 5 9 Describe the solution set as an inequality, in interval notation, and on a graph.
-
Each member of a large genetics class grows 12 pea plants from an independent pea family. Each family is expected to have 3/4 plants with smooth peas and 1/4 of the plants with wrinkled peas. On...
-
Verify that each of the terms in the sum-of-squares function (see Equation 9.9 on page 208) Sb y0 y ( y0 Xb ( b0 X0 y b0 X0 Xb is (1 1), justifying writing Sb y0 y ( 2y0 Xb b0 X0 Xb
-
The average age of Senators in the 114th congress was 61.7 years. If the standard deviation was 10.6, find the z scores of a senator who is 48 years old and one who is 66 years old.
-
An individual taxpayer has the gains and losses shown below. There are $4,000 of 1231 lookback losses. What is the net long-term capital gain? What is the net ordinary gain? Holding Period/Property...
-
Walton Ltd. is considering replacing an existing machine with a new and faster machine that will produce a more reliable product (i.e., better tolerances). The switch to a new machine resulting in a...
-
Starting with aniline, outline a synthesis of each of the following: (a) p-Bromoaniline (b) o-Bromoaniline (c) 2-Bromo-4-nitroaniline (d) 4-Bromo-2-nitroaniline
-
Starting with toluene, outline a synthesis of each of the following: (a) m-Chlorobenzoic acid (b) p-Methylacetophenone (c) 2-Bromo-4-nitrotoluene (d) p-Bromobenzoic acid (e)...
-
Consider the Excel file Mobiles Usage, which shows the number of people using different kinds of mobile phones in the northern region. Find the proportion of BlackBerry and Android usage in that...
-
Ja-San Company was started on January 1,2007, when the owners invested \($160,000\) cash in the business. During 2007, the company earned cash revenues of \($90,000\) and incurred cash expenses of...
-
Write a program using the programming language of your choice to implement the representation you designed for Review Question 3.3. Have your program solve the problem, and have it show on the screen...
-
All the lenses in Figure P33.98 are surrounded by air. Which of the lenses are converging, and which are diverging? Data from Figure P33.98 A B C D E F )(II)
-
Change the Growth and GrowthDriver classes described in the Improved Accuracy and Efficiency. Using a Step-with-Midpoint Algorithm subsection. Run your modified program with these inputs: For your...
-
For the three-element series circuit in Fig. 9-39, (a) Find the current I; (b) Find the voltage across each impedance and construct the voltage phasor diagram which shows that V 1 + V 2 + V 3 = 100 0...
-
Develop an argument for why a pharmaceutical firm should build and maintain an ethical climate.
-
Why do markets typically lead to an efficient outcome for buyers and sellers?
-
Explain why only one of the two chlorines of 1, 2-dichloro-2-methylpropane is replaced by a hydroxy group when the compound is heated in water (see the preceding hydrolysis reaction.
-
On the basis of the bond cleavage shown for this reaction in Figure 10.1, predict the stereo chemistry of the product.Explain. OCCH, CH,CH, ." -
-
Show the products of thesereactions: CI CH3CO, NaOH a) DMSO . Br CH,CO, b) DMF CH
-
Required information Skip to question [ The following information applies to the questions displayed below. ] Forten Company's current year income statement, comparative balance sheets, and...
-
Give a breakdown of all the intangible assets with the values shown in the statement of financial position of Unilever in 2022.
-
1-The yield to maturity will be greater than the coupon rate when a bond is selling at a premium. Select one: a. False b. True 2-Which one of the following would have the greatest present value,...

Study smarter with the SolutionInn App


