When farnesol is treated with sulfuric acid, it is converted to bisabolene. Outline a possible mechanism for
Question:
When farnesol is treated with sulfuric acid, it is converted to bisabolene. Outline a possible mechanism for this reaction.
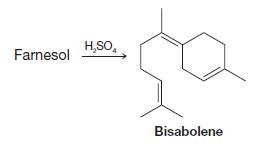
Transcribed Image Text:
Farnesol H₂SO4 Bisabolene
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 81% (11 reviews)
Solution The proposed mechanism for farnesol to bisabolene ...View the full answer

Answered By

Labindao Antoque
I graduated in 2018 with a Bachelor of Science degree in Psychology from Dalubhasaan ng Lungsod ng San Pablo. I tutored students in classes and out of classes. I use a variety of strategies to tutor students that include: lecture, discussions about the subject matter, problem solving examples using the principles of the subject matter being discussed in class , homework assignments that are directed towards reinforcing what we learn in class , and detailed practice problems help students to master a concept. I also do thorough research on Internet resources or textbooks so that I know what students need to learn in order to master what is being taught in class .
0.00
0 Reviews
10+ Question Solved
Related Book For 

Organic Chemistry
ISBN: 978-1118875766
12th Edition
Authors: T. W. Graham Solomons, Craig B. Fryhle, Scott A. Snyder
Question Posted:
Students also viewed these Sciences questions
-
When 2-methyl-2-propanol is treated with sulfuric acid, 2-methylpropene is formed. Show all of the steps in the mechanism for this reaction. Don't forget to use curved arrows to show the movement of...
-
A possible mechanism for the pathology of trinucleotide repeat diseases is aberrant translation in which all three reading frames are used. Which repeating amino acid residues will result from the...
-
A possible mechanism for a gas-phase reaction is given below. What is the rate law predicted by this mechanism? (fast equilibrium) 2NOCI NOC NO2NOCI (slow)
-
Suppose that you are holding your toy submarine under the water. You release it and it begins to ascend. The graph models the depth of the submarine as a function of time. What is the domain and...
-
Repeat Exercise 29, but let n be the number of collisions (in thousands) for the year that is t years since 1985. Which of your responses for this exercise are the same as those for Exercise 29?...
-
Which pdfs would have the following moment generating functions? (a) MY(t) = e6t2 (b) MY(t) = 2/(2 t) (c) MX(t) = (1/2 + 1/2 et)4 (d) MX(t) = 0.3et/(1 0.7et)
-
Discuss types of software available to assist in project stakeholder management? LO.1
-
The demand for subassembly S is 100 units in week 7. Each unit of S requires 1 unit of T and 2 units of U. Each unit of T requires 1 Unit of V, 2 units of W, and 1 unit of X. Finally, each unit of U...
-
DLW Corporation acquired and placed in service the following assets during the year: Asset Date Acquired Cost Basis Computer equipment 3/5 $ 18,500 Furniture 4/22 $ 25,900 Commercial building 8/19 $...
-
The number of friends reported by Facebook users is summarized in the following frequency distribution: FRIENDSf 400 above ...... 2 350 399...... 5 300 349....... 12 250 299....... 17 200 ...
-
When morphine reacts with 2 mol of acetic anhydride, it is transformed into the highly addictive narcotic heroin. What is the structure of heroin?
-
One of the important steps in Gates synthesis of morphine involved the following transformation: Suggest how this step was accomplished. CHO CHO NC CHO CHO NC-
-
Let G be an abelian group. Show that the elements of finite order in G form a subgroup. This subgroup is called the torsion subgroup of G.
-
Solve these question in details and fully explaination. It is the pre-lab working for Capacitors. Thanks so much in advance. 1: The figure shows a circuit with a charged capacitor (left), two...
-
Exercise 10-14A (Algo) Straight-line amortization of a bond discount LO 10-4 Diaz Company issued bonds with a $112,000 face value on January 1, Year 1. The bonds had a 8 percent stated rate of...
-
1. What would we have to plot on the vertical axis? EXPLAIN YOUR ANSWER OR NO CREDIT. [Hint: Solve for k first.] F= Kx kx dala you Experi determine the K= K = F Cart SHOW ALL WORK OR NO CREDITI 2....
-
You are interested in computing the heat transfer properties of a new insulation system shown here. Tair Air Layer 1 Layer 2 T P
-
16.3 The demand function for replicas for the Statue of Liberty is given by f(p): = 500 - 2p, where f(p) is the number of statues that can be sold for p dollars. (a) What is the relative rate of...
-
Nu-Look Design, Inc., operated as a residential home improvement company. During calendar years 1996, 1997, and 1998, Ronald A. Stark not only was Nu-Looks sole shareholder and president but also...
-
A liquid flows upward through a valve situated in a vertical pipe. Calculate the differential pressure (kPa) between points A and B. The mean velocity of the flow is 4.1 m/s. The specific gravity of...
-
Predict the multiplicity of the absorption for Hm if Jam = Jmx. Explain. , , . -C-C-C-
-
Construct a tree diagram for the absorption of Hm assume that Jam . . - -C-
-
Predict the multiplicities of the absorptions for the hydrogen's of these groups, assume that hydrogen's labeled a are different from those labeled x but that all of those labeled a are identical and...
-
Suppose you took a long position on a put option with an exercise price of $2.15 per pound and paid a premium of $0.24 per pound. Required: If the spot exchange rate turns out to be $2.30 per pound...
-
Youve observed the following returns on Crash-n-Burn Computers stock over the past five years: 15 percent, 6 percent, 18 percent, 14 percent, and 10 percent. Suppose the average inflation rate over...
-
Required : a- outline the statement of comperhensive income for the year ended 30 november 2021 b- outline the statment of financial position as at 30 November The Trial Balance of Alim Enterprise at...

Study smarter with the SolutionInn App


