(a) Write reactions to show how you could convert 2-methyl-2-butene into 2-methyl-1,3-butadiene. (b) Write reactions to show...
Question:
(a) Write reactions to show how you could convert 2-methyl-2-butene into 2-methyl-1,3-butadiene.
(b) Write reactions to show how you could convert ethylbenzene into the following compound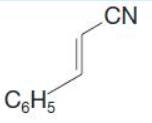
(c) Write structures for the various Diels–Alder adduct(s) that could result in reaction of 2-methyl-1,3-butadiene with the compound shown in part (b).
Transcribed Image Text:
C6H5 CN
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 64% (17 reviews)
a The reaction involves an intramolecular addition of ...View the full answer

Answered By

Brian Otieno
I'm Brian , an experienced professional freelancer with countless hours of success in freelancing many subjects in different disciplines. Specifically, I have handled many subjects and excelled in many disciplines. I have worked on many Computer Science projects and have been able to achieve a lot in that field. Additionally, I have handled other disciplines like History, Humanities, Social Sciences, Political science, Health care and life science, and Religion / Theology. My experience generally in these subjects has made me able to deliver high-quality projects in a very timely fashion. I am very reliable at my job and will get the work done in time, no matter what. In Addition, I have managed to ensure that the work meets my client's expectations and does not cause an error. I am a hard-working and diligent person who is highly responsible for everything I do. Generally, Freelancing has made me more accountable for doing my job. Additionally, I have had a passion for writing for the last seven years in this field.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Organic Chemistry
ISBN: 978-1118875766
12th Edition
Authors: T. W. Graham Solomons, Craig B. Fryhle, Scott A. Snyder
Question Posted:
Students also viewed these Sciences questions
-
Write reactions to show how nitric and sulfuric acids are produced in the atmosphere. Write reactions to show how the nitric and sulfuric acids in acid rain react with marble and limestone. (Both...
-
Show how you could convert the ethyl ester of Z-Phe-Gly to Leu-Phe-Gly (as its ethyl ester) by the active ester method.
-
Show how you could prepare each of the following compounds. Use the starting material indicated along with ethyl acetoacetate or diethyl malonate and any necessary inorganic reagents. Assume also...
-
Q8 Question: 9 A small particle of mass m moving inside a heavy, hollow and straight tube along the tube axis undergoes elastic collision at two ends. The tube has no friction and it is closed at one...
-
3(x 2y) = 9 Determine the slope and the y-intercept. Use the slope and the y-intercept to graph the equation by hand.
-
How do the risk categories in the risk-based capital model for property-casualty insurance companies differ from those of life insurance companies? What are the assumed relationships between the risk...
-
2. Prove that each of the following functions is uniformly continuous on (0,1). (a) (b) (c) (d) (e) x3 -1 f(x) = --1' x- . 1 f(x) = xsm-. x f(x) is any polynomial. f(x) = sin x . x
-
On December 31, 2015, analysis of Sayer Sporting Goods' operations for 2015 revealed the following. (a) Total cash collections from customers, $105,260. (b) December 31, 2014, inventory balance,...
-
A cost that has already been paid regardless of whether a capital budgeting project is accepted or rejected is known as a/an Group of answer choices opportunity cost direct agency cost indirect...
-
Leggere, an internet book retailer, is interested in better understanding the purchase decisions of its customers. For a set of 2,000 customer transactions, it has categorized the individual book...
-
Predict the products of the following reactions. (a) (b) HCI
-
Elucidate the structure of the compound that gives the following spectroscopic data. Assign the data to specific aspects of your proposed structure. MS (m/z); 120, 105 (base peak), 77 1 H NMR ():...
-
Arctic Charm Corp. is a privately owned Canadian company. The company experienced poor operating results in the years 20X0 to 20X3, and, in 20X3, it reorganized and refinanced its operations....
-
Aircraft \(B\) has a constant speed of \(150 \mathrm{~m} / \mathrm{s}\) as it passes the bottom of a circular loop of 400-m radius. Aircraft \(A\) flying horizontally in the plane of the loop passes...
-
A small inspection car with a mass of \(200 \mathrm{~kg}\) runs along the fixed overhead cable and is controlled by the attached cable at \(A\). Determine the acceleration of the car when the control...
-
An aircraft \(P\) takes off at \(A\) with a velocity \(v_{0}\) of \(250 \mathrm{~km} / \mathrm{h}\) and climbs in the vertical \(y^{\prime}-z^{\prime}\) plane at the constant \(15^{\circ}\) angle...
-
If each resistor in Figure P31.75 has resistance \(R=5.0 \Omega\), what is the equivalent resistance of the combination? Data from Figure P31.75 wwwwww wwwww www www wwwww
-
Identify the proper point to recognize expense for each of the following transactions. a. Kat Inc. purchases on credit six custom sofas for \(\$ 800\) each in June. Two of the sofas are sold for \(\$...
-
Lululemon has expanded its global retail operations through company-owned stores. In what instances might it consider using joint ventures as its global market entry strategy into new markets?
-
On January 2, 20X3, Sheldon Bass, a professional engineer, moved from Calgary to Edmonton to commence employment with Acco Ltd., a large public corporation. Because of his new employment contract,...
-
Suggest a mechanism for thisreaction: CH3 CH, CH3 CH3 N. CHCI CI
-
In addition to the reaction shown on p. 353, Diphenhydramine can also be prepared by heating bromo diphenyl methane and 2-(dimethyl lamino)-1-ethanol in a polar solvent. Show a mechanism for...
-
Another Diphenhydramine synthesis is shown in the following equation: (a) Show a mechanism for the first step in this synthesis. (b) Explain which mechanism is occurring in the secondstep. OCH CH...
-
Machinery is purchased on May 15, 2015 for $120,000 with a $10,000 salvage value and a five year life. The half year convention is followed. What method of depreciation will give the highest amount...
-
Flint Corporation was organized on January 1, 2020. It is authorized to issue 14,000 shares of 8%, $100 par value preferred stock, and 514,000 shares of no-par common stock with a stated value of $2...
-
Question 24 Not yet answered Marked out of 1.00 P Flag question Muscat LLC's current assets and current liabilities are OMR 258,000 and OMR 192,000, respectively. In the year 2020, the company earned...

Study smarter with the SolutionInn App


