In addition to the reaction shown on p. 353, Diphenhydramine can also be prepared by heating bromo
Question:
In addition to the reaction shown on p. 353, Diphenhydramine can also be prepared by heating bromo diphenyl methane and 2-(dimethyl lamino)-1-ethanol in a polar solvent. Show a mechanism for thisreaction:
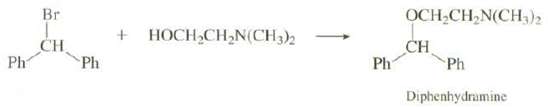
Transcribed Image Text:
Br OCH,CH,N(CH,)2 HOCH CH,N(CH3)2 CH Ph CH Ph Ph Ph Diphenhydramine
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 56% (16 reviews)
Ph Br CH Ph SN1 ...View the full answer

Answered By

Issa Shikuku
I have vast experience of four years in academic and content writing with quality understanding of APA, MLA, Harvard and Chicago formats. I am a dedicated tutor willing to hep prepare outlines, drafts or find sources in every way possible. I strive to make sure my clients follow assignment instructions and meet the rubric criteria by undertaking extensive research to develop perfect drafts and outlines. I do this by ensuring that i am always punctual and deliver quality work.
5.00+
6+ Reviews
13+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Diphenhydramine can also be synthesized by heating bromo diphenyl methane with the amino alcohol shown here. Offer a reason why the oxygen, rather than the nitrogen, of this compound acts as the...
-
In addition to the FASB's statement on accounting for research and development activities, the EITF has addressed three implementation issues. List and briefly summarize each of these issues.
-
In addition to the proton marked Ha in m-nitrostyrene in Figure 13.18, there are two other vinylic protons. Assuming that the coupling constant between the two geminal protons in ArCHoeCH2 is 2 Hz...
-
What is the type of the expressions computed on these two lines? 4 > 5 print (4>5)
-
For each of the costs listed below, indicate whether it is: (a) A product or period cost (b) A variable or fixed cost (c) A manufacturing or non-manufacturing cost. 1. Advertising costs of Nike. 2....
-
Golden Corporation is considering the purchase of new equipment costing $200,000. The expected life of the equipment is 10 years. It is expected that the new equipment can generate an increase in net...
-
Plot the six risks on a probability/impact matrix, using Figure 11-5 as a guide. Also assign a numeric value for the probability and impact of each risk on meeting the main project objective. Use a...
-
Stewart Beauf is a self-employed surfboard maker in 2018. His Schedule C net income is $125,003 for the year. He also has a part-time job and earns $15,600 in wages subject to FICA taxes. Calculate...
-
The value of an unlevered firm is equal to: EBIT + (1 T C )] / R U . [EBIT (1 T C )] / R U . V L + T C D. V L (T C / D).
-
Jimmy owns a garden in which he has planted N trees in a row. After a few years, the trees have grown up and now they have different heights. Jimmy pays much attention to the aesthetics of his...
-
Suggest a mechanism for thisreaction: CH3 CH, CH3 CH3 N. CHCI CI
-
Another Diphenhydramine synthesis is shown in the following equation: (a) Show a mechanism for the first step in this synthesis. (b) Explain which mechanism is occurring in the secondstep. OCH CH...
-
The monthly mobile phone bill for all customers at a large telecommunications company is found to be normally distributed with a mean of $145.55 per month and standard deviation of $15.22 per month....
-
Complete the exercises on the following website. Remember to type your answers in word or excel, screenshot, or phone pic as your work. The site does not save your answers. Upload your work on...
-
There are many management theories that are utilized in organizations. These theories were developed by scholars in the management discipline. One individual was responsible for identifying the major...
-
An increase in the price and a decrease of the quantity of Paclitaxel (an anti-cancer drug) could be caused by which of the following? Select one: O a. an increase in the number of people being...
-
At December 31, 2023, Cord Company's plant asset and accumulated depreciation and amortization accounts had balances as follows: Category Land Land improvements Buildings Equipment Automobiles and...
-
Assume that the following table represents the sales figures for the five largest firms in the industry. Compute the HHI for the industry (assuming the industry contains just these five firms). Sales...
-
Outline the main preoccupations of qualitative researchers.
-
Determine two different Hamilton circuits in each of the following graphs. A B F G
-
Solve each system by graphing. 2.x +3y = 6 4.x + 6.y = 12 -4 -3 in # 3 10 + || + 15 fet en - HAT
-
Draw an energy diagram for the three molecular orbitals of the cyclopropenyl system (C3H3). How are these three molecular orbitals occupied in the cyclopropenyl anion, cation, and radical? Which of...
-
Cyclopropanone is highly reactive because of its large amount of angle strain, but methylcyclopropenone, although even more strained than Cyclopropanone, is nevertheless quite stable and can even be...
-
Cycloheptatrienone is stable, but cyclopentadienone is so reactive that it can?t be isolated. Explain, taking the polarity of the carbonyl group into account. Cycloheptatrienone Cyclopentadienone
-
Suppose you took a long position on a put option with an exercise price of $2.15 per pound and paid a premium of $0.24 per pound. Required: If the spot exchange rate turns out to be $2.30 per pound...
-
Youve observed the following returns on Crash-n-Burn Computers stock over the past five years: 15 percent, 6 percent, 18 percent, 14 percent, and 10 percent. Suppose the average inflation rate over...
-
Required : a- outline the statement of comperhensive income for the year ended 30 november 2021 b- outline the statment of financial position as at 30 November The Trial Balance of Alim Enterprise at...

Study smarter with the SolutionInn App


