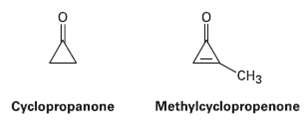
Cyclopropanone is highly reactive because of its large amount of angle strain, but methylcyclopropenone, although even more
Question:
Cyclopropanone is highly reactive because of its large amount of angle strain, but methylcyclopropenone, although even more strained than Cyclopropanone, is nevertheless quite stable and can even be distilled. Explain, taking the polarity of the carbonyl group intoaccount.
Transcribed Image Text:
"CHз Cyclopropanone Methylcyclopropenone
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 54% (11 reviews)
00 A 8 0 CH3 B CH3 In resonance structure A methylcyclopropenone is a cyclic conjugated com...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
The polarization of a carbonyl group can be represented by a pair of resonance structures: Cyclopropenone and cycloheptatrienone are more stable than anticipated. Cyclopentadienone, however, is...
-
Explain why, for more than a decade, a massive amount of money flowed into the United States. Compare and contrast your explanation with that of the President. Most economists agree that the problems...
-
Because a center equilibrium is stable but not asymptotically stable, nonlinear perturbation can have different outcomes. shown in Problems I 1 and 12. 1. Determine the stability of the equilibrium...
-
Great ride (GR) is in the business of manufacturing and selling high-end vehicles. GR signs a deal with the CEO of a consulting firm for a luxury SUV. You are the long-time Controller for GreatRide...
-
What challenges does the system pose for drivers and their managers?
-
Georgia McBeal is trying to save for her retirement. She believes she can earn 10% on average each year on her retirement fund. Assume that at the beginning of each of the next 40 years, Georgia will...
-
Jodi Jackson is the general manager of a student-operated public restaurant in a hospitality management program at a large state university. She used her old dish machine for as long as she could,...
-
Ace Inc. has five employees participating in its defined-benefit pension plan. Expected years of future service for these employees at the beginning of 2014 are as follows. Future Employee Years of...
-
A company's employees had the following earnings records on June 30, the current payroll period: Employees Earnings Earnings this FICA FICA Income Med Net Pay FUTAI FUTA SUTA through Prior Pay Period...
-
This problem is based on the JA Tires data that was first introduced in problem 6-35. If you have not already accessed the data, it can be downloaded from the textbook website. As part of risk...
-
Draw an energy diagram for the three molecular orbitals of the cyclopropenyl system (C3H3). How are these three molecular orbitals occupied in the cyclopropenyl anion, cation, and radical? Which of...
-
Cycloheptatrienone is stable, but cyclopentadienone is so reactive that it can?t be isolated. Explain, taking the polarity of the carbonyl group into account. Cycloheptatrienone Cyclopentadienone
-
How does roll forging differ from a conventional rolling operation?
-
Kelly Corporation received an advanced payment of \(\$ 30,000\) in 2018 from Rufus Company for consulting services. Kelly performed half of the consulting in 2018 and the remainder in 2019. Kelly...
-
Rosa Dominguez, the owner of Elegant Dining in San Jose, California, is pondering whether to buy electronic menu technology and tablets for her five-star restaurant. Prices for a typical four course...
-
Dura Corporation makes metal frames for several world brands of portable home generators. They sell the frames to a wide variety of portable generator manufacturers such as DeWalt, DuroMax, Generac,...
-
Rocker Industries (RI) produces recreational in-line skates (see Exhibit 14.36). Demand is seasonal, peaking in the summer months, with a smaller peak demand during December. For one of their more...
-
The BOM, current inventory, and lead time (in months) for the in-line skates in Rocker Industries (A) case is shown in Exhibit 14.37. Using the chase demand strategy, you developed in Rocker...
-
define an associated company and understand the main accounting issues involved in accounting for an investment in an associated company.
-
Calculate Total Contribution Margin for the same items. Total Revenue Total Variable Costs Total Contribution Margin $50.00 a. $116.00 $329.70 b. $275.00 $14,796.00 $7,440.00 c. $40,931.25 d....
-
Nitrogen monoxide, a pollutant in automobile exhaust, is oxidized to nitrogen dioxide in the atmosphere according to the equation: Find K c for this reaction. 2 NO(g) + 0(8) 2 NO(8) Kp 2.2 x 102 at...
-
Suggest a method for the synthesis of the unnatural D enantiomer of alanine from the readily available L enantiomer of lactic acid. CH,-CHOH-COOH lactic acid
-
Show how you would use the Gabriel-malonic ester synthesis to make histidine. What stereochemistry would you expect in your synthetic product?
-
Show how you would use the Strecker synthesis to make tryptophan. What stereochemistry would you expect in your synthetic product?
-
Jennifer purchased a home for $1,000,000 in 2016. She paid $200,000 cash and borrowed the remaining $800,000. This is Jennifer's only residence. Assume that in year 2024, when the home had...
-
business plan describing company with strengths and weaknesses. Any gaps in plan. Recommendations for improvement of the plan.
-
You wish to buy a car today for $35,000. You plan to put 10% down and finance the rest at 5.20% p.a. for six years. You will make equal monthly payments of $_______.

Study smarter with the SolutionInn App


