Question: Give formulas and names for compounds A and B: -5a-Cholest-2-ene CHCOOH A (an epoxide) HBr B
Give formulas and names for compounds A and B: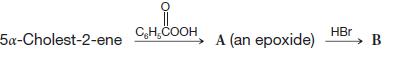
-5a-Cholest-2-ene CHCOOH A (an epoxide) HBr B
Step by Step Solution
★★★★★
3.37 Rating (150 Votes )
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock

Answer and thorough explana... View full answer

Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


