When the following molecule was exposed to the Grubbs I initiator at different concentrations, different products resulted.
Question:
When the following molecule was exposed to the Grubbs I initiator at different concentrations, different products resulted. How can you account for these different outcomes?
(a)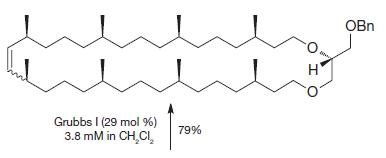
(b)
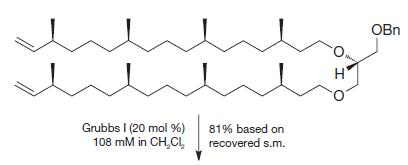
(c)
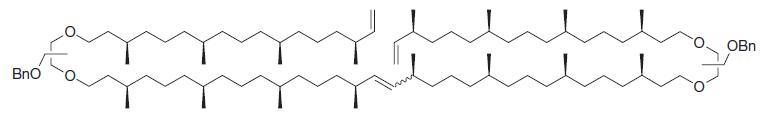
Transcribed Image Text:
Grubbs I (29 mol %) 3.8 mM in CH₂Cl₂ 79% O I OBn
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (12 reviews)
The different products that were formed when the molecule was exposed to th...View the full answer

Answered By

BillClinton Muguai
I have been a tutor for the past 5 years. I have experience working with students in a variety of subject areas, including computer science, math, science, English, and history. I have also worked with students of all ages, from elementary school to college. In addition to my tutoring experience, I have a degree in education from a top university. This has given me a strong foundation in child development and learning theories, which I use to inform my tutoring practices.
I am patient and adaptable, and I work to create a positive and supportive learning environment for my students. I believe that all students have the ability to succeed, and it is my job to help them find and develop their strengths. I am confident in my ability to tutor students and help them achieve their academic goals.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Organic Chemistry
ISBN: 978-1118875766
12th Edition
Authors: T. W. Graham Solomons, Craig B. Fryhle, Scott A. Snyder
Question Posted:
Students also viewed these Sciences questions
-
How can you account for the fact that 2, 2, 6-trimethylcyclohexanone yields no detectable aldol product even though it has an acidic hydrogen?
-
How can you account for the fact that cis-1, 3-pentadicne is much less reactive than trans-1, 3-pentadiene in the DielsAlder reaction?
-
The following molecule was founded by an intermolecular aldol reaction. What dicarbonyl precursor was used for itspreparation?
-
The AND function can be realized by using only n number of NOR gates. The value of nequal to
-
The loudness of sound can be measured by using a decibel scale. Some examples of sounds at various sound levels are listed in Table 55. The sound level of music from a Pioneer MT-2000 stereo-...
-
What is the coefficient of x12 in the expansion of (1+x3 +x6)18?
-
Describe the contents of a stakeholder management plan? LO.1
-
Let's see whether quadratic voting can avoid the paradox of voting that arose in Table 5.3 when using 1p1v in a series of paired-choice majority votes. To reexamine this situation using quadratic...
-
Imagine that A La Mode Pies, a company that sells pies, has $500,000 of sales in November and has a contribution margin ratio of 29% and fixed costs of $80,000. If sales in units increase by 8% in...
-
A survey conducted by the Northwestern University Lindquist-Endicott Report asked 320 companies about the procedures they use in hiring. Only 54% of the responding companies review the applicants...
-
Draw the structure of a phospholipid (from any of the subclasses of phospholipids) that contains one saturated and one unsaturated fatty acid. (a) Draw the structure of all of the products that would...
-
Gramin has been synthesized by heating a mixture of indole, formaldehyde, and dimethylamine. (a) What general reaction is involved here? (b) Outline a reasonable mechanism for the gramine synthesis.
-
Match the organizations on the left with the functions on the right. Each function should be used only once. Organization Function Institute of Internal Auditors a. The group that creates and...
-
Let f(x) = x+ 3, x20. The inverse of f is Of 1(x)=x - 3 (f (x) = -x-3 f-(x) = x - 3 Of 1(x) = 3 - x
-
Read the articles and please help me to write the whole assignment perfectly including the citations and references (APA Format). Pleaase choose the country and perspective of a particular industry....
-
A light, inextensible cord passes over a frictionless pulley as shown in figure below. One end of the rope is attached to a block, and a force P is applied to the other end. Block A weighs 600 lb and...
-
BASICOT POST DO NOT ASSIST DO NOT POST DO NOT ASSIST DO NOT POST DO NOT ASSIST For filming a physics demonstration about oscillation, an educational video crew attaches a large spring to a very small...
-
Day Mail Order Co. applied the high-low method of cost estimation to customer order data for the first 4 months of the year. What is the estimated variable order-filling cost component per order...
-
Emory B. Perry, who represents numerous shareholders, owned stock of The RAMP Corporation, a now-defunct company that developed communications technologies for the health care industry. Darryl R....
-
Use multiplication or division of power series to find the first three nonzero terms in the Maclaurin series for each function. y = e x2 cos x
-
Suggest a possible structure for a compound with the formula C7H12O that has the following IR spectrum and explain yourreasoning: 80 40 20 1718 cm 0- 500 1000 2000 1500 2500 3000 3500 4000 Wavenumber...
-
Suggest a possible structure for a compound with the formula C9H10O that has the following IR spectrum and explain yourreasoning: 100 - 50 1706 em- 4000 3500 3000 2500 2000 1000 500 1500 Wavenamber...
-
Forensic laboratories often have to identify various illicit drug samples. Explain how IR spectroscopy could be used to help distinguish between morphine and heroin. CH3CO, . NH NH CH,CO" ...
-
nformation pertaining to Noskey Corporation s sales revenue follows: November 2 0 2 1 ( Actual ) December 2 0 2 1 ( Budgeted ) January 2 0 2 2 ( Budgeted ) Cash sales $ 1 0 5 , 0 0 0 $ 1 1 5 , 0 0 0...
-
The management team of Netflix maintains a stable dividend using the Lintner model: Dt+1 = Dt + EPS Target Payout Where Dt (Dt+1) = dividend in the current period t (the next period t + 1) EPSt =...
-
#1 #2 hapter 50 10 D Werences lav Help Required information [The following information applies to the questions displayed below) Archer Company is a wholesaler of custom-built air-conditioning units...

Study smarter with the SolutionInn App


