The 1H NMR signals for the aromatic hydrogens of methyl p-hydroxybenzoate appear as two doublets at approximately
Question:
The 1H NMR signals for the aromatic hydrogens of methyl p-hydroxybenzoate appear as two doublets at approximately 7.05 and 8.04 ppm (δ). Assign these two doublets to the respective hydrogens that produce each signal. Justify your assignments using arguments of relative electron density based on contributing resonance structures.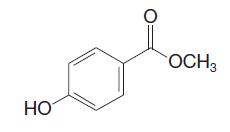
Transcribed Image Text:
HO 。 OCH3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 73% (15 reviews)
My argument on my assignment is as follows i The chemical shift of the downfield ...View the full answer

Answered By

Brian Otieno
I'm Brian , an experienced professional freelancer with countless hours of success in freelancing many subjects in different disciplines. Specifically, I have handled many subjects and excelled in many disciplines. I have worked on many Computer Science projects and have been able to achieve a lot in that field. Additionally, I have handled other disciplines like History, Humanities, Social Sciences, Political science, Health care and life science, and Religion / Theology. My experience generally in these subjects has made me able to deliver high-quality projects in a very timely fashion. I am very reliable at my job and will get the work done in time, no matter what. In Addition, I have managed to ensure that the work meets my client's expectations and does not cause an error. I am a hard-working and diligent person who is highly responsible for everything I do. Generally, Freelancing has made me more accountable for doing my job. Additionally, I have had a passion for writing for the last seven years in this field.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Organic Chemistry
ISBN: 978-1118875766
12th Edition
Authors: T. W. Graham Solomons, Craig B. Fryhle, Scott A. Snyder
Question Posted:
Students also viewed these Sciences questions
-
Order the 1H NMR signals of the following compounds by chemical-shift position (lowest to highest). Which one is the most upheld? The most downfield? (a) H3C-CH3 (b) H2C==CH2 (c) H3C-O-CH3 (d) (e)...
-
The 1H NMR signal for bromoform (CHBr3) appears at 2065 Hz when recorded on a 300-MHz NMR spectrometer. (a) What is the chemical shift of this proton? (b) Is the proton in CHBr3 more shielded or less...
-
The 1H NMR signal for bromoform (CHBr3) appears at 2065 Hz when recorded on a 300-MHz NMR spectrometer. (a) What is the chemical shift of this proton? (b) Is the proton in CHBr3 more shielded or less...
-
Compare and contrast megaloblastic anemia caused by vitamin B12 deficiency and that caused by folic acid deficiency. (10)
-
Let M be the median (in points) and R be the range (in points) of four test scores (all in points), x1, x2, x3, and x4, where the scores are listed from smallest to largest. a. Write a formula for...
-
Defend the statement that although off-balance-sheet activities expose FIs to several forms of risks, they also can alleviate the risks of FIs.
-
9. Prove that a polynomial of degree n is uniformly continuous on R if and only if n = or 1.
-
Describe the layout of a typical fast-food franchise such as McDonalds. What type of layout is it? How does it support productivity? Do different franchises (e.g., Burger King or Wendys) have...
-
024. Abbroviation used. Dopr = depreciation ) Warehouse Supplies \begin{tabular}{l|l} \hline Bal 220 & \\ \hline & \end{tabular} \begin{tabular}{|l|c|} \hline \multicolumn{2}{c}{ Supplies...
-
the next two years. Currently, WSDC has $2,000,000 on hand and available for investment. In 6 months, 12 months, and 18 months, WSDC expects to receive an income stream from previous Winston-Salem...
-
The structure of thyroxine, a thyroid hormone that helps to regulate metabolic rate, was determined in part by comparison with a synthetic compound believed to have the same structure as natural...
-
The following reaction sequence was used by E. J. Corey (J. Am. Chem. Soc. 1969, 91, 56755677) at the beginning of a synthesis of prostaglandin F 2 and prostaglandin E 2 . Explain what is involved in...
-
What are the three categories of business analytics?
-
n1 = 20, n2 = 25, S = 607, H1: 1 2. In Exercises 710, compute S, S, and the value of the test statistic z. Then find the P-value for the specified alternate hypothesis and values of n1, n2, and S.
-
To determine whether traffic levels differ between the morning and evening rush hours, a traffic engineer counted the number of cars passing through a certain intersection during five-minute periods...
-
Macon Timber Company established a \(\$ 150\) petty cash fund on January 1, 2012. Required a. Is the establishment of the petty cash fund an asset source, use, or exchange transaction? b. Record the...
-
Following is a bank reconciliation for Holt's Sandwich Shop for May 31, 2012: Because of limited funds, Holt's employed only one accountant who was responsible for receiving cash, recording receipts...
-
For each of the following situations, fill in the blank with FIFO, LIFO, or weighted average. a. b. c. d. e. f. would produce the highest amount of net income in an inflationary environment. would...
-
Explain how the use of sustainability scorecard could add value to a company in the eyes of its consumers.
-
Using Gauss-Jordan elimination, invert this matrix ONLY 0 0 0 0 1
-
Explain whether these elimination reaction would be a good way to prepare thesealkenes: Cl H,O CH,OH + KOH CI EIOH PHCH=CHCH, b) PHCH CHCH; + NaOEt
-
Explain which of these reactions would provide a better synthesis of2-pentene: Br CH,OH CH,CH,CHCH,CH, + CH;0 CH,CH=CHCH,CH3 Br CH, CH CH=CHCH CH, CH,CHCH,CH,CH; + CH,0
-
Show the products of thesereactions: Br CH,OH CH3CH,CHCH,CH, + CH,0 CH,CH-CHCH,CH3 Br CH;OH CH,CHCH,CH,CH; + CH,0 CH.CH-CHCH,CH; CH, CH-CH2 2 b) ELOH Br Br
-
. Emerson Cammack wishes to purchase an annuity contract that will pay him $7,000 a year for the rest of his life. The Philo Life Insurance Company figures that his life expectancy is 20 years, based...
-
Integrity Inc. can sell 20-year, $1,000 par value bonds paying semi-annual interests with a 10% coupon. The bonds can be sold for $1,050 each; flotation cost of $50 per bond will be incurred in this...
-
Duncan Inc. issued 500, $1,200, 8%, 25 year bonds on January 1, 2020, at 102. Interest is payable on January 1. Duncan uses straight-line amortization for bond discounts or premiums. INSTRUCTIONS:...

Study smarter with the SolutionInn App


