Papaverine has been synthesized by the following route: Outline the reactions involved. CHO CHO NH CHO CHO
Question:
Papaverine has been synthesized by the following route:
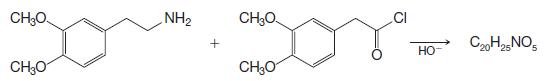

Outline the reactions involved.
Transcribed Image Text:
CH₂O CH₂O NH₂ CH₂O CH₂O CI НО- C20H25 NO5
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 63% (11 reviews)
Solution The reaction that takes place is as follows In the first step hydroxyl OH ions are form...View the full answer

Answered By

Labindao Antoque
I graduated in 2018 with a Bachelor of Science degree in Psychology from Dalubhasaan ng Lungsod ng San Pablo. I tutored students in classes and out of classes. I use a variety of strategies to tutor students that include: lecture, discussions about the subject matter, problem solving examples using the principles of the subject matter being discussed in class , homework assignments that are directed towards reinforcing what we learn in class , and detailed practice problems help students to master a concept. I also do thorough research on Internet resources or textbooks so that I know what students need to learn in order to master what is being taught in class .
0.00
0 Reviews
10+ Question Solved
Related Book For 

Organic Chemistry
ISBN: 978-1118875766
12th Edition
Authors: T. W. Graham Solomons, Craig B. Fryhle, Scott A. Snyder
Question Posted:
Students also viewed these Sciences questions
-
The sex hormone estrone has been synthesized by a route that involves the following step. Identify the pericyclic reactions involved, and propose amechanism. CH CH Heat |CH30 CH0 Estrone methyl ether
-
Gramin has been synthesized by heating a mixture of indole, formaldehyde, and dimethylamine. (a) What general reaction is involved here? (b) Outline a reasonable mechanism for the gramine synthesis.
-
The sedative-hypnotic ethinamate can be synthesized by the following route. Provide structures for ethinamate and the intermediates A and B: Cyclohexanone (1) HC=CNa, (2) HO+ A (CH12O) CICOCI B...
-
Due to improved technology and public-service campaigns, the number of collisions at highwayrailroad crossings per year has declined since 1992 (see Table 52). Let n be the number of collisions (in...
-
One of the popular tourist attractions in Alaska is watching black bears catch salmon swimming upstream to spawn. Not all black bears are black, though some are tan-colored. Suppose that six black...
-
Suppliers and concerned citizens are examples of which type of stakeholders? a. internal b. external c. supportive d. unsupportive LO.1
-
Use the information in RES-7. Calculate Uncle Butchs Hunting Supply Shops ending inventory' using the retail inventory method under the lower of average cost or market assumption. Round the...
-
Introduction to Finance ( 2 0 2 4 - 2 5 / S 2 ) Assessment: Online Task Weightage: 1 0 % ANSWER THE FOLLOWING QUESTIONS AND UPLOAD YOUR SUBMISSION IN MOODLE IN EITHER PDF OR WORD FORMAT TCM is...
-
1. Despite all the changes that have happened with the Internet since 2007, when Songkick was founded, why do you think the company has been able to successfully stick with its original business...
-
One of the important steps in Gates synthesis of morphine involved the following transformation: Suggest how this step was accomplished. CHO CHO NC CHO CHO NC-
-
Many alkaloids appear to be synthesized in plants by reactions that resemble the Mannich reaction. Recognition of this (by R. Robinson in 1917) led to a synthesis of tropinone that takes place under...
-
How can you use the volume of some quantity of a pure substance to calculate its mass?
-
1. Gluteus maximus muscle and mention the structures covered by it.
-
2. External features and relations of Kidney.
-
3. Features of Synovial joint and its classification.
-
1. Name the paranasal air sinuses.
-
2. Name the ventricles of Brain.
-
William Roberts operated a McDonalds restaurant under a franchise agreement with McDonalds Corporation. Roberts hired 23-year-old David Mabin, who was just released from jail for robbery, drug use,...
-
A Bloomberg Businessweek subscriber study asked, In the past 12 months, when traveling for business, what type of airline ticket did you purchase most often? A second question asked if the type of...
-
Determine the structure of this compound from its IR and 13C-NMR spectra, its formula isC7H16O2: C,H1,0, 80- 60- %T 40 20- 0- 500 1000 1500 2000 2500 3500 3000 4000 Wavenumber (cm) C,H1602 All CH's...
-
Predict the multiplicities of the indicated hydrogen's in the 1H-NMR spectra of these compounds: CH,CI I H I - + CH,CI CI - c) b) --- I H I - ()
-
Predict the approximate chemical shifts, multiplicities and integrals for the absorptions in the 1H-NMR spectra of thesecompounds: CH2 b) .- COCH CH3 a) d) CH;CHCH CH3 c) CH;CH,NHCH,CH3
-
Columbus Industries makes a product that sells for $37 a unit. The product has a $29 per unit variable cost and total fixed costs of $10,000. At budgeted sales of 1,950 units, the margin of safety...
-
18. Suppose that Maxima shares are selling for $10 per share and you own a call option to buy Maxima shares at $7.50. The intrinsic value of your option is:
-
ABC Insurance Company reported the following information on its accounting statements last year: What was ABC 's expense ratio last year

Study smarter with the SolutionInn App


