Predict the outcome of the following reactions: CN CI 2 equiv. KNH lig. NH3, 33 C
Question:
Predict the outcome of the following reactions:
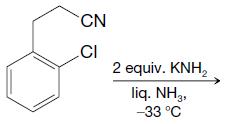
Transcribed Image Text:
CN CI 2 equiv. KNH₂ lig. NH3, 33 C
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (5 reviews)
For the first reaction the expected outcome is the formation of 1hexanol This is because the reactan...View the full answer

Answered By

BillClinton Muguai
I have been a tutor for the past 5 years. I have experience working with students in a variety of subject areas, including computer science, math, science, English, and history. I have also worked with students of all ages, from elementary school to college. In addition to my tutoring experience, I have a degree in education from a top university. This has given me a strong foundation in child development and learning theories, which I use to inform my tutoring practices.
I am patient and adaptable, and I work to create a positive and supportive learning environment for my students. I believe that all students have the ability to succeed, and it is my job to help them find and develop their strengths. I am confident in my ability to tutor students and help them achieve their academic goals.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Organic Chemistry
ISBN: 978-1118875766
12th Edition
Authors: T. W. Graham Solomons, Craig B. Fryhle, Scott A. Snyder
Question Posted:
Students also viewed these Sciences questions
-
Predict the outcome of the reactions represented by the following equations by using the activity series, and balance the equations. (a) Cu(s) + HCl(aq) (b) 12(s) + NaBr(aq)- (c) Mg(s) + CuSO (aq...
-
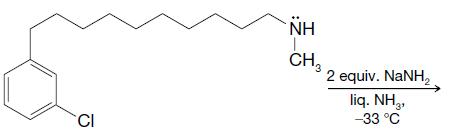
Predict the outcome of the following reactions: (a) (b) CN CI 2 equiv. KNH2 liq. NH3 -33 C NH CH3 2 equiv. NaNH liq. NH2 -33C Cl
-
Predict the outcome of the following reaction. -NH2
-
The Kc for the following reaction is 9.30 X 10^-2 at 25C:PCl5(g) <-> PCl3(g) + Cl2(g) How many moles & grams of PCl5 must be added to a 2-literflask to obtain a Cl2 concentration of 0.150M...
-
X 3 1 Describe the solution set as an inequality, in interval notation, and on a graph.
-
Estimate the convexity for each of the following three bonds, all of which trade at yield to maturity of 8 percent and have face values of $1,000. A 7-year, zero-coupon bond. A 7-year, 10 percent...
-
*A crucial step in the proof of the Gauss-Markov theorem (Section 9.3.2) uses the fact that the matrix product AX must be 0 because AXfl 0. Why is this the case? [Hint: The key here is that AX 0...
-
What actions have CoachUp and Charity:Water taken to establish trust and credibility with various groups?
-
Question 4 On March 1, 2015, Newton Company purchased and for an office site by paying $540,000 cash. Newton began construction on the office building on March 1. The following expenditures were...
-
Grete Ropewalk formed a dog grooming and training business called Grete Koninis on September 1, 2014. After consulting with a friend who had taken introductory accounting, Grete created a chart of...
-
Provide a mechanism for the following reaction. NO NO 2 ss-sr NaOCH3 OH NO O=5 S=0 NO
-
Starting with aniline, outline a synthesis of each of the following: (a) p-Bromoaniline (b) o-Bromoaniline (c) 2-Bromo-4-nitroaniline (d) 4-Bromo-2-nitroaniline
-
On January 1, 2014, Garrison Corp, issued 13% bonds dated January 1,2014, with a face amount of $212 million. The bonds mature December 31, 2023. For bonds of similar risk and maturity, the market...
-
In an air-pollution study performed at an experiment station, the following amount of suspended benzenesoluble organic matter (in micrograms per cubic meter) was obtained for eight different samples...
-
The figure shows a sketch of the curve with equation y = f(x). The curve passes through the points (0, 3) and (4, 0) and touches the x-axis at the point (1, 0). On separate diagrams, sketch the...
-
An object is placed \(200 \mathrm{~mm}\) from a diverging thin lens that has a focal length of \(-500 \mathrm{~mm}\). What are (a) the image distance and \((b)\) the magnification? (c) Draw a...
-
In a study of warp breakage during the weaving of fabric (Technometrics [1982]: 63), 100 pieces of yarn were tested. The number of cycles of strain to breakage was recorded for each yarn sample. The...
-
Many consider family-owned businesses the backbone of American business. Mei Mei translates from Chinese to little sister in English, and its name aptly represents a family business of three...
-
A clothing company gives generously to charities and sponsors donation drives to help lower-income teen girls get reasonably priced prom dresses. It also locates its manufacturing plants in countries...
-
Conduct a VRIO analysis by ranking Husson University (in Maine) business school in terms of the following six dimensions relative to the top three rival schools. If you were the dean with a limited...
-
How might the structure of DDT be modified to make it again effective against resistant insects?
-
Explain which product is formed when each of these alkyl chlorides reacts with sodium ethoxide in ethanol.
-
Explain which of these alkyl chlorides reacts faster with sodium ethoxide in ethanol.
-
If the auditor believes that the financial statements prepared on the basis of the entity's income tax are not adequately titled, the auditor should : A)Issue a resignation of opinion. B)Explain the...
-
initial stock offering to the public. This REIT specializes in the acquisition and management of warehouses. Your firm, Blue Street Advisors, is an investment management company that is considering...
-
Question 3 You have been hired to run a pension fund for Mackay Inc, a small manufacturing firm. The firm currently has Gh5 million in the fund and expects to have cash inflows of $2 million a year...

Study smarter with the SolutionInn App


