In general, intramolecular reactions that form rings are often disfavored entropically because it makes a flexible starting
Question:
In general, intramolecular reactions that form rings are often disfavored entropically because it makes a flexible starting material more rigid. In the case of ring-closing metathesis, however, the entropy penalty for the closure is not especially large for most systems, such as the one below used as part of a synthesis of an anti-tumor compound known as epothilone A. Why might that be?
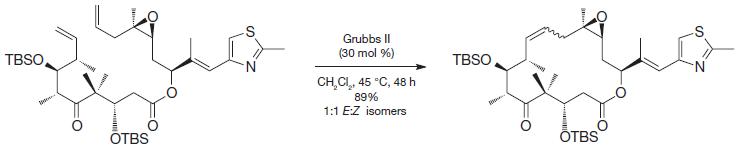
Transcribed Image Text:
TBSO THESE OTBS Grubbs II (30 mol %) CH,CI,, 45 °C, 48h 89% 1:1 E:Z isomers TBSO w OTBS N
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 71% (14 reviews)
It is because of two factors 1 metathesis reactions proceed via a ringopening step which is much more acidic than a ring closing step and 2 the final product is a macrocycle which will be rigid the strain energy required to form a macrocycle is higher than that of an open chain molecule In addition one must also consider the driving force for the reaction G The G term overall comes from two components the enthalpy change of the metathesis reaction ...View the full answer

Answered By

Brian Otieno
I'm Brian , an experienced professional freelancer with countless hours of success in freelancing many subjects in different disciplines. Specifically, I have handled many subjects and excelled in many disciplines. I have worked on many Computer Science projects and have been able to achieve a lot in that field. Additionally, I have handled other disciplines like History, Humanities, Social Sciences, Political science, Health care and life science, and Religion / Theology. My experience generally in these subjects has made me able to deliver high-quality projects in a very timely fashion. I am very reliable at my job and will get the work done in time, no matter what. In Addition, I have managed to ensure that the work meets my client's expectations and does not cause an error. I am a hard-working and diligent person who is highly responsible for everything I do. Generally, Freelancing has made me more accountable for doing my job. Additionally, I have had a passion for writing for the last seven years in this field.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Organic Chemistry
ISBN: 978-1118875766
12th Edition
Authors: T. W. Graham Solomons, Craig B. Fryhle, Scott A. Snyder
Question Posted:
Students also viewed these Sciences questions
-
A compound pendulum is defined as a rigid slab which oscillates about a fixed point O, called the center of suspension. Show that the period of oscillation of a compound pendulum is equal to the...
-
A spiro ring junction is one where two rings that share no bonds originate from a single carbon atom. Alkanes containing such a ring junction are called spiranes. (a) For the case of bicyclic...
-
Why should 3-methylcyclohexene not be used as the starting material in Problem 30b?
-
Create a T-Account Transaction Account titles Cash d Common stock Supplies Creditors accounts payable) Cash Fees earned Rent expense Cash Creditors(accounts payable) Cash Accounts receivable Fees...
-
Willie Mays, with all-around talent, was one of the greatest baseball players of all time. The numbers of stolen bases by Mays are shown in Table 77 for various years. Let n be the number of stolen...
-
After some further analysis, you discover that the commission field in the Policies table is updated yearly to reflect changes in the annual commission paid to agents on existing policies. Would...
-
The stereotype is that women are more concerned about their weight than men are concerned about their own weight. Is this true across the education spectrum? Using the PewHealth dataset, run a...
-
Explain how unethical business practices degrade the quality of the experience a customer has with a service or product. How is the International Organization for Standardization trying to encourage...
-
In the future, Blockchain applications will include; Select one: A. Only transactions involving cryptocurrencies B. Only transactions within the borders of one country C. Only transactions between...
-
The accompanying diagram shows the demand and supply curves for taxi rides in New York City. a. At E 1 the market is at equilibrium with 600 million miles of rides transacted at an equilibrium price...
-
The tranquilizing drug meprobamate (Equanil or Miltown) can be synthesized from 2-methylpentanal as follows. Give structures for meprobamate and for the intermediates AC: HCHO, HO H [A (C.H,102)]...
-
The tranquilizing drug meprobamate (Equanil or Miltown) can be synthesized from 2-methylpentanal as follows. Give structures for meprobamate and for the intermediates AC: H HCHO, HO [A (C,H,O,)]...
-
Herold had been a calendar year S corporation since 1990. On October 10, 2023, Mrs. Hughes sold 18 shares of Herold stock to a foreign partnership, thereby terminating Herolds S election. Herolds...
-
A company which manufactures microwaves advertises that 90% of their microwaves are flawless, requiring no adjustments. Their quality control department tests this percentage on a regular basis. On...
-
A new retail store is being planned for a site that contains 40 ft of soft clay (c 0.075 ft2/day, y = 100 pcf). The clay layer is overlain by 15 ft of sand (y = 112 pcf) and is underlain by dense...
-
Perez Bags (PB) is a designer of high-quality backpacks and purses. Each design is made in small batches. Each spring, PB comes out with new designs for the backpack and for the purse. The company...
-
Find a recent (within the last 12 months) article or economic blog related to price fixing, provide an executive summary of the information. Include an APA reference and/or link. How does the fact...
-
A rectangular block of a material with a modulus of rigidity G=90 ksi is bonded to two rigid horizontal plates. The lower plate is fixed, while the upper plate is subjected to a horizontal force P....
-
BZD, a calendar year corporation, made the following year-end accruals for 2016 financial statement purposes. In each case, determine how much of the accrued expense is deductible on BZDs 2016...
-
If a force of F = 50 Ib is applied to the pads at A and C, determine the smallest dimension d required for equilibrium if the spring has an unstretched length of 1 ft. B 1 ft 1 ft F k = 15016/fr 1ft...
-
Limonene, a major component of lemon oil, has the formula C10H16. (a) On reaction with excess H2 in the presence of Pt, limonene produces C10H20. What information does this provide about the...
-
Show the structures of A, B, C, and D in the following reactionsscheme: D Optically inactive H,SO. H,O B Pt C,H14 C,H12 Optically Optically inactive active 1) Hg(O,CCH3)2, H20 2) NaBH4, NAOH...
-
In Figure, suppose Br2 adds to the alkene from the bottom, rather than from the top as shown. Analyze the stereochemistry of the reaction in this case and explain which products areformed. Relative...
-
Suppose the S&P 500 currently has a level of 960. One contract of S&P 500 index futures has a size of $250 S&P 500 index. You wish to hedge an $800,000-portfolio that has a beta of 1.2. (A)In order...
-
Exhibit 4.1 The balance sheet and income statement shown below are for Koski Inc. Note that the firm has no amortization charges, it does not lease any assets, none of its debt must be retired during...
-
Haley is 57 years of age. She is planning for future long-term care needs. She knows that yearly nursing home costs in her area are currently $69,000, with prices increased by 5 percent annually....

Study smarter with the SolutionInn App


