Limonene, a major component of lemon oil, has the formula C10H16. (a) On reaction with excess H2
Question:
Limonene, a major component of lemon oil, has the formula C10H16.
(a) On reaction with excess H2 in the presence of Pt, limonene produces C10H20. What information does this provide about the structure of limonene?
(b) On ozonolysis, limonene produces these compounds. Suggest possible structures forlimonene.
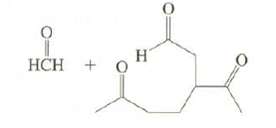
Transcribed Image Text:
Н НСН
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (10 reviews)
a The DU of limonene is 3 The DU of the hydrogena...View the full answer

Answered By

Branice Buyengo Ajevi
I have been teaching for the last 5 years which has strengthened my interaction with students of different level.
4.30+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
A major component of financial planning is to forecast future financial statements. If you had a company's balance sheets and income statements for the past 5 years but no other information, how...
-
A major component of gasoline is octane (c8h18). When liquid octane is burned in air it reacts with oxygen (o2) gas to produce carbon dioxide gas and water vapor. Calculate the moles of oxygen needed...
-
1- Explain why all the amino acids except for glycine are chiral? 2-Identify the amino acids that differ from each other by a single methyl or methylene group.? 3-Classify the 20 standard amino acids...
-
According to the American Red Cross, 11.6% of all Connecticut residents have Type B blood. A random sample of 28 Connecticut residents is taken. X = the number of Connecticut residents that have Type...
-
Grania knows that the pH of blood is normally 7.35 to 7.45. She sees that her blood test results show 7.57. Is Granias blood too acid or too alkaline or normal?
-
Road construction contracts in the state of Florida Are awarded on the basis of competitive, sealed bids; the contractor who submits the lowest bid price wins the contract. During the 1980s, the...
-
Assuming that n1 = n,, find the sample sizes nceded to estimate (p, - p2) for each of the following situations: a. Bound = .O1 with 99% confidence. Assume that p, = .4 and p, = .7. b. A 90%...
-
Mike Polanski is 30 years of age and his salary next year will be $40,000. Mike forecasts that his salary will increase at a steady rate of 5% per annum until his retirement at age 60. a. If the...
-
After the success of the company's first two months, Santana Rey cont / nues to operate busmess unadjusted trial balance of Business Solutions ( reflecting its transactions for October and Novemb...
-
1. Snyders of Hanover, which sells about 80 million bags of pretzels, snack chips, and organic snack items each year, had its financial department use spreadsheets and manual processes for much of...
-
Suggest a mechanism for thisreaction: CH,Br Br2 CH2=CHCH CH,CH,OH H,O
-
Show the structures of A, B, C, and D in the following reactionsscheme: D Optically inactive H,SO. H,O B Pt C,H14 C,H12 Optically Optically inactive active 1) Hg(O,CCH3)2, H20 2) NaBH4, NAOH...
-
On an exchange floor, what is a floor broker?
-
Revisits scope, time, and cost baselines in the context of agile methodologies. Because agile includes several methodologies (like Scrum, Kanban, Extreme Programming, Feature-Driven Development) we...
-
Background information and task: Fed officials divided in July over need for more rate hikes, minutes show WASHINGTON, Aug 16 (Reuters) - Federal Reserve officials were divided over the need for more...
-
Questions: 1. What are the long-term prospects for the Chinese market? 2. Does it make sense for GM to produce automobiles for the Chinese market in China? Why? 3. What do you think would happen if...
-
The purpose of this assignment is to apply your knowledge of conflict management to a real-world situation so that you can enhance your skills in handling conflicts. It is crucial to carefully read...
-
Roy's Toys received a shipment of 100,000 rubber duckies from the factory. The factory couldn't promise that all rubber duckies are in perfect form, but they promised that the percentage of defective...
-
Why should employee discharge be viewed as a last-resort disciplinary step?
-
Suppose that the electrical potential at the point (x, y, z) is E(x, y, z) = x + y - 2z. What is the direction of the acceleration at the point (1,3,2)?
-
A popular chemical demonstration is the magic genie procedure, in which hydrogen peroxide decomposes to water and oxygen gas with the aid of a catalyst. The activation energy of this (uncatalyzed)...
-
The octapeptide angiotensin II has the sequence Asp-Arg-Val-Tyr-IIe-His-Pro-Phe. What fragments would result if angiotensin II were cleaved with trypsin with chymotrypsin?
-
What is the N-terminal residue on a peptide that gives the following PTH derivative on Edmandegradation?
-
Draw the structure of the PTH derivative that would he formed on Edman degradation of angiotensin II (Problem 26.12).
-
If the auditor believes that the financial statements prepared on the basis of the entity's income tax are not adequately titled, the auditor should : A)Issue a resignation of opinion. B)Explain the...
-
initial stock offering to the public. This REIT specializes in the acquisition and management of warehouses. Your firm, Blue Street Advisors, is an investment management company that is considering...
-
Question 3 You have been hired to run a pension fund for Mackay Inc, a small manufacturing firm. The firm currently has Gh5 million in the fund and expects to have cash inflows of $2 million a year...

Study smarter with the SolutionInn App


