Predict the product from each of the following reactions. (a) (b) (c) (d) heat
Question:
Predict the product from each of the following reactions.
(a)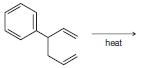
(b)
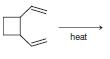
(c)
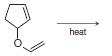
(d)
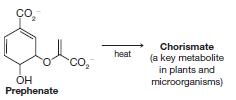
Transcribed Image Text:
heat
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (8 reviews)
The major product of the each reaction is given below A It is an ex...View the full answer

Answered By

LEONARD KIPRUTO
I am Complete M.Sc chemistry from YCIS Satara and qualified UGC CSIR NET-JRF with AIR-26 also qualified GATE 2022 and SET from MAHARASHTRA and able to solve many questions of chemistry like competitive exams.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Organic Chemistry
ISBN: 978-1118875766
12th Edition
Authors: T. W. Graham Solomons, Craig B. Fryhle, Scott A. Snyder
Question Posted:
Students also viewed these Sciences questions
-
Predict the major product from each of the following reactions. (a) (b) (c) (d) (e) (f) OH SOC, pyr OH HBr NaNH2 OH OH (1) TsCI, pyr (2) EtSNa Nal, H2SO OH
-
Predict the organic product from each of the following oxidation reactions. (a) (b) (c) (d) (e) (1) KMnO4, HO. OH (2) H,O OH PCC CH2Cl2 OH (1) DMSO, (COCI)2 (2) EtgN OH H2CrO H2CrO
-
Predict the organic product from each of the following oxidation and reduction reactions. (a) (b) (c) (d) (e) HO HCrO4 OH HO OH 1) LAH (2) aq. H2SO4 NaBH4
-
Shauna immigrated to Canada in her 30s and has worked full time earning above YMPE throughout. Now she is a few years from retirement and has found out that her monthly OAS at age 65 will be $520....
-
The scatterplot and linear model in Fig. 96 describe the prices of hot dogs and soft drinks at all Major League Baseball (MLB) stadiums. a. Use the linear model to predict the soft drink price at an...
-
Show that the expected value associated with the exponential distribution, fY (y) =e y, y>0, is 1/, where is a positive constant.
-
When should you start controlling stakeholder engagement on a project? a. in the early phases b. in the middle phases c. in the latter phases d. none of the above; you cannot control stakeholder...
-
Colorado Mountain Mining paid $507,700 for the right to extract mineral assets from a 500,000-ton deposit. In addition to the purchase price, Colorado also paid a $600 filing fee, a $1,700 license...
-
8. Because electronic commerce technology advances have the potential to greatly reduce the time delays involved in processing payments and invoices, many trading partners (buyers and sellers) are...
-
The R&D department is planning to bid on a large project for the development of a new communication system for commercial planes. The accompanying table shows the activities, times, and sequences...
-
What reactant could lead to each product by either a Cope or Claisen rearrangement? (a) (b) ? heat
-
When compound A is heated, compound B can be isolated from the reaction mixture. A sequence of two electrocyclic reactions occurs; the first involves a 4-electron system, and the second involves a...
-
If a company reported the following items on its income statement (cost of goods sold $6,000, income tax expense $2,000, interest expense $500, operating expenses $3,500, sales revenue $14,000), what...
-
?In civil engineering, what is the main use of a slump test in concrete technology?
-
Briefly explain what spatial autocorrelation means and what method can be used to measure it
-
Discuss the theoretical implications of adopting biodegradable materials in civil engineering for reducing environmental impact and enhancing sustainability.
-
Analyze the role of civil engineering in coastal erosion management. What are the engineering strategies for shoreline protection, beach nourishment, and coastal infrastructure design to mitigate...
-
Analyze the impact of climate change on civil engineering practices, particularly in the areas of coastal and floodplain management, and discuss strategies for mitigating these impacts
-
Donald Lynch purchased a check from Allied Irish Bank (AIB) in Ireland. The check was made payable to Advance Marketing and Investment Inc. (AMI) in the amount of U.S. $250.00, which was handwritten...
-
What is the order p of a B + -tree? Describe the structure of both internal and leaf nodes of a B + -tree.
-
Trans-1-Phenyl-1, 3-butadiene has max = 280 ( = 27,000) calculate the concentration of a solution that has A = 0.643 at 280nm in a 1 cm cell.
-
Nitro methane max = 275nm ( = 1.5) what kind of transition is responsible for this absorption?
-
3-Buten-2-one has max =213nm ( = 7080) and max = 320nm ( = 21) what kind of transition is responsible for each of these absorptions?
-
An estimated 84 percent of enterprises now use cloud computing solutions involving multiple clouds, whereas less than 10 percent of large organizations employ just a single public cloud. Group of...
-
XYZ inc. was involved in a tax dispute with the national tax authority. The companys legal counsel estimates that there is a 75% likelihood that the company will lose the dispute and that the amount...
-
3 . Accounting.. How does depreciation impact financial statements, and what are the different methods of depreciation?

Study smarter with the SolutionInn App


