Write the equilibrium expression (K) for each of the following gas-phase reactions. a. N(g) + O(g)=2NO(g) b.
Question:
Write the equilibrium expression (K) for each of the following gas-phase reactions.
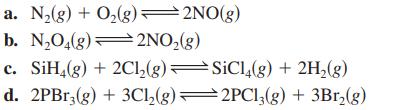
Transcribed Image Text:
a. N₂(g) + O₂(g)=2NO(g) b. N₂O₂(g)2NO₂(g) c. SiH4(g) + 2Cl₂(g) d. 2PBr3(g) + 3Cl₂(g) SiCl4(g) + 2H₂(g) 2PC13(g) + 3Br₂(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (5 reviews)

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry An Atoms First Approach
ISBN: 9781305079243
2nd Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl
Question Posted:
Students also viewed these Sciences questions
-
Consider a gas phase reaction where N2O4 (g) 2NO2 (g) at 25C. The equilibrium constant (Kp) for the reaction is 0.25 atm. a) Write the equilibrium expression for Kp. b) Calculate the partial...
-
A stirred tank with volume V t? (L) is charged with V 1 (L) of a liquid. B. The space above the liquid (volume V g = V t ? V 1 ) is filled with a pure gas, A, at an initial pressure P 0 (atm). The...
-
Give the expression for K for each of the following reactions. a. b. c. CaCO (s)CaO(s) CO2(g) Pbl2(s) H (a) HCO (aq)H2O) CO2(g)
-
A slipper-pad bearing (Fig. P1023) is often encountered in lubrication problems. Oil flows between two blocks; the upper one is stationary, and the lower one is moving in this case. The drawing is...
-
What If the Facts Were Different? Suppose that shortly before Pallisters death, she had asked James to tear up her will, and he had done it. Would the result have been different? Explain.
-
A chemist needs a buffer with pH 4.35. How many milliliters of pure acetic acid (density = 1.049 g/mL) must be added to 465 mL of 0.0941 M NaOH solution to obtain such a buffer?
-
Describe the basic types of supervisory skills.
-
Detroit Synthetic Fibers, Inc., specializes in the manufacture of synthetic fibers used in many products such as blankets, coats, and uniforms. The company applies overhead on the basis of...
-
Can someone please help me with my homework 7. (4 points) M Company recorded entries for (1) issuing common stock for $250,000, (2) collecting $100,000 of accounts receivable, (3) incurring, but not...
-
Using the accompanying Retirement Calculator spreadsheet model, Claire wants to use Scenario Manager to compare the following retirement saving scenarios: Click here for the Excel Data File Scenario...
-
Consider the following reaction at a certain temperature: An equilibrium mixture contains 1.0 mole of Fe, 1.0 10 -3 mole of O 2 , and 2.0 moles of Fe 2 O 3 all in a 2.0-L container. Calculate the...
-
For a typical equilibrium problem, the value of K and the initial reaction conditions are given for a specific reaction, and you are asked to calculate the equilibrium concentrations. Many of these...
-
You are valuing a young technology company that is experiencing high growth. What income approach model will you likely use? Explain.
-
Locate a scholarly article relevant to how to present your financial plan for opening a Roller Skating Rink (from your draft business plan) to a lending institution--and describe your strategy for...
-
How would you expect seasonal fluctuations in demand to affect a rental company's decisions about pricing rented products such as wedding dresses or convertible cars? In terms of pricing principles,...
-
Do we drive technology, or does technology drive us? If technology drives us, what are the risks? The other side of the coin would be that we are able to stay ahead of technological transformations....
-
How do you explain the differences between the two analyses and what are the implications of using the BCG matrix in practice?
-
How do leadership styles, such as transformational leadership, shared leadership, and servant leadership, impact team dynamics, member motivation, and overall team effectiveness ?
-
Which party (union or management) would likely be in a stronger position to bargain for its preferred wage outcome under the following conditions and why?
-
What are the key elements of a system investigation report?
-
Assign a name for each of the following compounds: (a) (b) (c) (d) (e) (f) `NH2 NH2
-
Draw all constitutional isomers with molecular formula C 4 H 11 N, and provide a name for each isomer.
-
Draw all tertiary amines with molecular formula C 5 H 13 N, and provide a name for each isomer. Are any of these compounds chiral?
-
The following are selected transactions of PT Santorini. PT Santorini prepares financial statements quarterly: (all transactions are in millions) Feb 1 Purchase Merchandise on account from PT Ibiza...
-
Material B = 3.25 (1.500-1,400) = 3.25 (100) = Rs. 325 Material C = 3.50 (2,100 -2,000) = 3.50 (100) = Rs. 350 Material Usage Variance = Rs. 575 Favourable Verification: MCV = MPV + MUV - Rs.550:-...
-
Alpine Luggage has a capacity to produce 370,000 sultcases per year. The company is currently producing and selling 290,000 units per year at a selling price of $399 per case. The cost of producing...

Study smarter with the SolutionInn App


