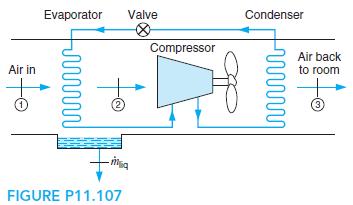
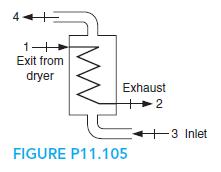
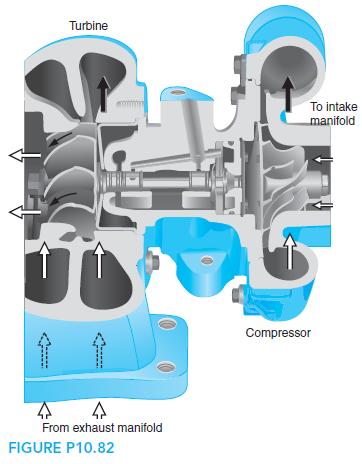
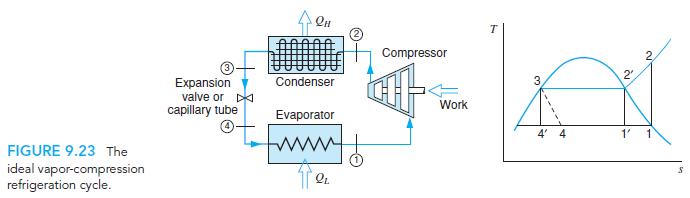
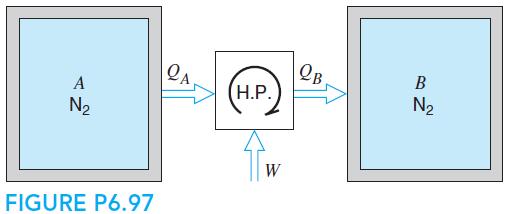
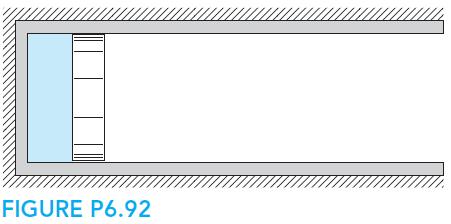
Fundamentals Of Thermodynamics 8th Edition Claus Borgnakke, Richard E. Sonntag - Solutions
Unlock a wealth of knowledge with "Fundamentals of Thermodynamics 8th Edition" by Claus Borgnakke and Richard E. Sonntag. Dive into a comprehensive collection of questions and answers, providing step-by-step solutions to enhance your understanding. Access our detailed solution manual and answers key, perfect for navigating complex solved problems. Benefit from our extensive test bank, featuring chapter solutions and instructor manuals tailored for thorough preparation. Whether you're seeking a textbook resource or solutions in PDF format, explore our free download options for convenient access to invaluable insights and guidance in thermodynamics.
![]()
![]() New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
![]()
![]()