Consider a gasifier that receives 4 kmol CO, 3 kmol H 2 , and 3.76 kmol N
Question:
Consider a gasifier that receives 4 kmol CO, 3 kmol H2, and 3.76 kmol N2 and brings the mixture to equilibrium at 900 K, 1 MPa, with the following reaction:
2 CO+ 2H2 ⇔ CH4 + CO2
which is the sum of Eqs. 14.32 and 14.33. If the equilibrium constant is K = 2.679, find the exit flow composition.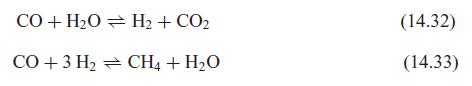
Transcribed Image Text:
CO + H20 = H2 + CO2 (14.32) CO + 3 H2 = CH4 + H20 (14.33)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (10 reviews)
To find the exit flow composition we need to calculate the extent of reaction and then use the equil...View the full answer

Answered By

Akshay Shete
I have extensive experience as a tutor, both online and in-person. I have worked with students of all ages and abilities, and am skilled at adapting my teaching style to meet the needs of each individual student. I have a strong background in a variety of subjects, including math, science, and English, and am able to break down complex concepts in a way that is easy for students to understand. In addition to my subject matter expertise, I am also a patient and supportive teacher, and am committed to helping my students succeed. Whether I am working with a struggling student who needs extra help to catch up, or an advanced student looking to get ahead, I am able to provide the guidance and support they need to reach their goals. Overall, my hands-on experience as a tutor has prepared me to be a confident and effective teacher, and I am excited to use my skills to help students succeed.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Fundamentals Of Thermodynamics
ISBN: 9781118131992
8th Edition
Authors: Claus Borgnakke, Richard E. Sonntag
Question Posted:
Students also viewed these Sciences questions
-
Consider an initial mixture of N2 and H2 gases that can be represented as follows. The gases react to form ammonia gas (NH3) as represented by the following concentration profile. a. Label each plot...
-
A mixture of 1 kmol of CO and 2 kmol of O2 is heated to 2000 K at a pressure of 2 atm. Determine the equilibrium composition of (a) O2, (b) CO2 (in kmols) assuming the mixture consists of CO2, CO and...
-
The equilibrium reaction with methane as CH4 C + 2H2 has ln K = -0.3362 at 1440 R and ln K = -4.607 at 1080 R. By noting the relation of K to temperature, show how you would...
-
A group of fraud examiners is coordinating an investigation at a local law firm. Several lawyers at the firm are suspected of overbilling clients, possibly creating fake client accounts, and then...
-
Required Prepare General Journal entries to record the following transactions of Billington Company. 2015 Jan. 10, Accepted a $3,000, 60-day, 6% note dated this day in granting a time extension on...
-
The cone-and-plate viscometer (see Fig. 2B.11). A cone-and-plate viscometer consists of a stationary flat plate and an inverted cone, whose apex just contacts the plate. The liquid whose viscosity is...
-
3. When Al DeAngelis started his new job as a computer programmer, he arrived in his department at 9:30 a.m., after having spent time in the human resources department filling out forms. Marcia...
-
On January 1, 2014, Cron Corporation issued $700,000 in bonds that mature in five years. The bonds have a stated interest rate of 13 percent and pay interest on June 30 and December 31 each year....
-
Forchen, Inc., provided the following information for two of its divisions for last year: Sales Operating income Operating assets, January 1 Operating assets, December 31 Small Appliances Cleaning...
-
Organizations often need to delve deep into culture in order to ensure a diverse and represented workplace. What can organizations do inorder to improve intercultural communication?, In your answer,...
-
A coal gasifier produces a mixture of 1 CO and 2H 2 that is fed to a catalytic converter to produce methane. This is the methanation reaction in Eq. 14.33 with an equilibrium constant at 600 K of K =...
-
Repeat the previous problem, assuming the argon constitutes 1% of a gas mixture where we neglect any reactions of other gases and find the pressure that will give a mole concentration of A r + of...
-
Earth can be described as a three-part energy system. Explain. What is meant by the term equilibrium surface temperature?
-
Before beginning a study investigating the ability of the drug heparin to prevent bronchoconstriction, baseline values of pulmonary function were measured for a sample of 12 individuals with a...
-
which of the following (list all that apply) are advantages of a balanced binary search tree over an unbalanced one: 1. it requires less memory 2. it's faster to move from node to node 3. it's faster...
-
6) Do you find conditional probability problems challenging? Have you tried watching the videos on canvas and has it helped?
-
1. Determine the cost of heating 3 gallons of water (water weighs 8.33L per gallon ) at a room temperature of 22 degrees Celsius to the boiling point of 100 degrees Celsius at the energy rating of...
-
Writer One Inc. manufactures ball point pens that sell at wholesale for $0.80 per unit. Budgeted production in both 2018 and 2019 was 16,000 units. There was no beginning inventory in 2018. The...
-
The following transactions occurred during June 2018 for Soulful Art, Inc.: Jun 3 Purchased $3,300 of goods on account. Terms, 2/10, n/30, FOB shipping point. 6 Returned $800 of defective merchandise...
-
What will be the final value of DI after executing the following piece of code? Execute the instructions dependently one after another. CLD MOU CX,OFOH MOU AX.02874H MOU DI,01000H MOU ES, DI SUB...
-
When aniline is treated with fuming sulfuric acid, an electrophilic aromatic substitution reaction takes place at the meta position instead of the para position, despite the fact that the amino group...
-
Para-Nitroaniline is an order of magnitude less basic than meta-nitroaniline. (a) Explain the observed difference in basicity. (b) Would you expect the basicity of ortho-nitroaniline to be closer in...
-
Methadone is a powerful analgesic that is used to suppress withdrawal symptoms in the rehabilitation of heroin addicts. Identify the major product that is obtained when methadone is subjected to a...
-
Cash from Operating Activities: ______________ Cash from Investing Activities: ______________ Cash from Financing Activities: ______________ Problem 2: Financial Ratios The GAP Macys 1 Current Ratio...
-
On January 1, 2021, Winky Enterprises issued 12% bonds dated January 1, 2021, with a face amount of $2,800,000. The bonds mature in 2030 (10 years). For bonds of similar risk and maturity, the market...
-
Using the following accounts and balances, prepare the stockholders' equity vection of the balance sheet. Pilty thousand shares of common stock are authorised, and 1,000 shares have been recoured,...

Study smarter with the SolutionInn App


