Verify that the ideal gas part of the Helmholtz function substituted in Eq. 12.86 does lead to
Question:
Verify that the ideal gas part of the Helmholtz function substituted in Eq. 12.86 does lead to the ideal gas law, as in the note after Eq. 12.97.
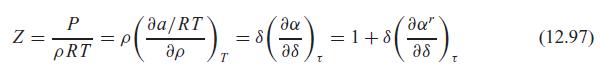
![]()
Transcribed Image Text:
da/RT da" = p pRT ap = 8 as =1+8 a8 (12.97) T
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 71% (14 reviews)
To verify that the ideal gas part of the Helmholtz function leads to the ideal gas law we can start ...View the full answer

Answered By

Joshua Marie Geuvara
I am an academic writer with over 5 years of experience. I write term papers, essays, dissertations, reports, and any other academic paper. My main objective is to produce a high-quality paper free from plagiarism and ensure a student scores an A+. Being a fluent English speaker, I have great communication skills that also enable me to produce excellent papers.
I am conversant with most academic referencing styles (APA, MLA, and Harvard).
You can trust me with your paper and expect nothing less than quality and excellent results. I look forward to meeting with you and, more importantly, developing something that will both make us happy and satisfied.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Fundamentals Of Thermodynamics
ISBN: 9781118131992
8th Edition
Authors: Claus Borgnakke, Richard E. Sonntag
Question Posted:
Students also viewed these Sciences questions
-
Assuming that the ideal gas law holds, find the amount of nitrogen gas in a container if P = 0.836 atm 0.003 atm, V = 0.01985 m 3 0.00008 m 3 , T = 298.3 K 0.2 K. Find the expected error in the...
-
The ideal gas law relates four variables. An empirical gas law relates two variables, assuming the other two are constant. How many empirical gas laws can be obtained? Give statements of each.
-
The gas law for an ideal gas at absolute temperature T (in kelvins), pressure P (in atmospheres), and volume V (in liters) is PV = nRT, where is the number of moles of the gas and R = 0.0821 is the...
-
A machine fills containers with a particular product. The standard deviation of filling weights computed from past data is 0.6 ounces. If only 2% of the containers hold less than 18 ounces, what is...
-
Suppose 1 = $0.0077 in London, $1 = SF2.00 in New York, and SF1= 65 in Paris. a. If you begin by holding 10,000 yen, how could you make a profit from these exchange rates? b. Find the arbitrage...
-
When a charged particle approaches (or leaves) a conducting surface, radiation is emitted, associated with the changing electric dipole moment of the charge and its image. If the particle has mass m...
-
9. I think that interactive multimedia software is the best form of training for everyone. True or False
-
Donelson Corporation was organized on January 1, 2010. It is authorized to issue 20,000 shares of 6%, $40 par value preferred stock, and 500,000 shares of no-par common stock with a stated value of...
-
Exercise 5-6 Break-Even Analysis (L05-5) Mauro Products distributes a single product, a woven basket whose selling price is $29 per unit and whose variable expense is $23 per unit. The company's...
-
Edit the program provided so that it receives a series of numbers from the user and allows the user to press the enter key to indicate that he or she is finished providing inputs. After the user...
-
R-410a is a 1:1 mass ratio mixture of R-32 and R-125. Find the specific volume at 20C, 1200 kPa, using Kays rule and the generalized charts, and compare it to the solution using Table B.4.
-
Gases like argon and neon have constant specific heats. Develop an expression for the ideal gas contribution to the Helmholtz function in Eq. 12.92 for these cases. a* = h* RT Ts* (12.92)
-
The diagram shows a sector OAB of a circle with centre O and radius r cm. The angle AOB is radians and the perimeter of the sector is 30 cm. a) Show that = 30/r 2. b) Find the area of the sector...
-
Dr. Burgess oversees the pharmacy center within Hughes Regional Hospital. Dr. Burgess is planning on purchasing two medication dispensing units which she wants to pay back in a short-term period. The...
-
On January 1, 2021, Wetick Optometrists leased diagnostic equipment from Southern Corp., which had purchased the equipment at a cost of $1,831,401. The lease agreement specifies six annual payments...
-
Prevosti Farms and Sugarhouse pays its employees according to their job classification. The following employees make up Sugarhouse's staff: Employee Whatis late and Address Payroll information A -...
-
Image caption
-
Jamie Lee and Ross, now 57 and still very active, have plenty of time on their hands now that the triplets are away at college. They both realized that time has just flown by, over twenty-four years...
-
The account balances for the year ended December 31, 2018, for River City Fabrication, Inc., are listed next: Requirements 1. Prepare River City Fabrication, Inc.?s multistep income statement. 2....
-
Name some of the various types of financial intermediaries described in the chapter and indicate the primary reason(s) each was created.
-
Rank the following compounds in terms of increasing basicity: N. Br z-
-
When (E)-4-amino-3-buten-2-one is treated with molecular hydrogen in the presence of platinum, the resulting amine is more basic than the reactant. Draw the reactant and the product, and explain why...
-
For each of the following compounds, draw the form that predominates at physiological pH: (a) (b) (c) CI CH CI Sertraline (Zoloft) An antidepressant NH2 Amantadine Used in the treatment of...
-
A government bond matures in 30 years, makes semi-annual coupon payments of 6.0% ($120 per year) and offers a yield of 3.7% annually compounded. Assume face value is $1,000. Three years later the...
-
Your objective is: 1. Carry out a life insurance needs analysis, for each one of them (show your calculations) [30 Marks] 2. Refer to the case and the insurance plan quotes. Would you recommend...
-
TufStuff, Incorporated, sells a wide range of drums, bins, boxes, and other containers that are used in the chemical industry. One of the company s products is a heavy - duty corrosion - resistant...

Study smarter with the SolutionInn App


