Two rigid tanks, shown in Fig. P6.97, each contains 10 kg N 2 gas at 1000 K,
Question:
Two rigid tanks, shown in Fig. P6.97, each contains 10 kg N2 gas at 1000 K, 500 kPa. They are now thermally connected to a reversible heat pump, which heats one tank and cools the other, with no heat transfer to the surroundings. When one tank is heated to 1500 K, the process stops. Find the final (P, T) in both tanks and the work input to the heat pump, assuming constant heat capacities.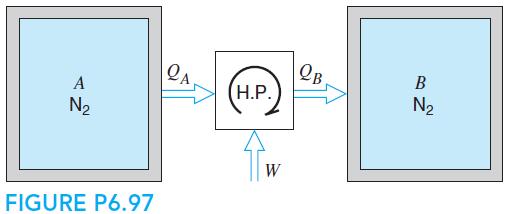
Transcribed Image Text:
OB H.P. A N2 N2 W FIGURE P6.97
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 72% (11 reviews)
Given the following parameter P A 1 P B1 T A1 T B1 T B2 Find the answers to these missing parameters ...View the full answer

Answered By

Saida kasyoka
I am an experienced writer with four years of work experience Over the course of four years, as a writer, I have completed more than 200 papers. I was pursuing a Diploma in Electrical/Electronics Engineering prior to becoming an academic/article writer. My favorite subjects to write about for essays are sociology, literature, psychology, political science, ethics, religion, and the English language. I'm very passionate about tutoring.
I enjoy looking for original ways to offer information, which is why I love tutoring. My audience and my aim will both influence the tone. Any tutoring I do should always have an objective—what I aim to achieve. Knowing who my audience is should be obvious from knowing what my purpose is, and those two things should be all I need to know what to offer my student.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Fundamentals Of Thermodynamics
ISBN: 9781118131992
8th Edition
Authors: Claus Borgnakke, Richard E. Sonntag
Question Posted:
Students also viewed these Sciences questions
-
Two rigid tanks each contain 10 kg N2 gas at 1000 K, 500 kPa. They are now thermally connected to a reversible heat pump, which heats one and cools the other with no heat transfer to the...
-
A mass-loaded piston/cylinder, shown in Fig P6.133, containing air is at 300 kPa, 17C with a volume of 0.25 m3, while at the stops V = 1 m3. An air line, 500 kPa, 600 K, is connected by a valve that...
-
A reversible heat pump uses 1 kW of power input to heat a 25oC room, drawing energy from the outside at 15oC. Assuming every process is reversible, what are the total rates of entropy into the heat...
-
Data-2-Go manufactures and sells flash drives. The company produces only when it receives orders and, therefore, has no inventories. The following information is available for the current month:...
-
"Monetary disequilibrium leads to balance of payments problems under fixed exchange rates, and a currency problem under floating exchange rates." Discuss this statement with reference to the monetary...
-
Calculate P(A; ) in Example 1 if the sample size is increased from n = 2 to n = 3, the other data remaining as before. Compute P (A; 0.10) and P(A; 0.20) and compare with Example 1.
-
Apply the present value of cost in cost comparison
-
In Problem S1-8, if Wiley Publishing is able to assign probabilities of occurrence of 0.23 to unfavorable market conditions, 0.46 for the same market conditions, and 0.31 for favorable market...
-
INFORMATION: FOR BUILDING >> Initial Investment $10,000,000 Annual Cash Inflows $1,250,000 Discount Rate 10% NPV = $864,920 Number of Years 20 Salvage Value $ 1,500,000 FOR EQUIPMENT >> Initial...
-
The triangular frame ABC can be adjusted vertically between the three equal-length cords. If it remains in a horizontal plane, determine the required distance s so that the tension in each of the...
-
Two rigid, insulated tanks are connected with a pipe and valve. One tank has 0.5 kg air at 200 kPa, 300K and the other has 0.75 kg air at 100 kPa, 400 K. The valve is opened, and the air comes to a...
-
A hydrogen gas in a piston/cylinder assembly at 300 K, 100 kPa with a volume of 0.1 m 3 is now slowly compressed to a volume of 0.01 m 3 while cooling in a reversible isothermal process. What is the...
-
The Green river Manufacturing Company manufactures two products: Raft and Float. At December 31, 2012, Green river used the FIFO inventory method. Effective January 1, 2013, Green river changed to...
-
A retail product has the following standard costs established: Direct Material per unit - 2 pounds at $5 a pound Direct Labor per unit - 3 hours at $12 an hour Manufacturing Overhead - $5 per labor...
-
In a recent year, the Better Business Bureau settled 75% of complaints they received. (Source: USA Today, March 2, 2009) You have been hired by the Bureau to investigate complaints this year...
-
A 1200-ft equal tangent crest vertical curve is currently designed for 50 mph. A civil engineering student contends that 60 mph is safe in a van because of the higher driver's eye height. If all...
-
Required information [The following information applies to the questions displayed below.] Victory Company uses weighted-average process costing to account for its production costs. Conversion cost...
-
Finer, % 100 90 80 70 60 50 40 30 20 10 0 0.01 0.1 1 Size, mm L 10 100 Figure shows a grain size distribution curve of soil. Estimate the coefficient of curvature (Cc) of this soil.
-
Tom Rasta engaged in the following activities in establishing his photography studio, Picture This! Indicate the accounts to be debited and credited for each transaction. 1. Opened a bank account in...
-
B made an issue of 150,000 $1 ordinary shares at a premium of 20% the proceeds of which is received by cheque. What is the correct journal to record this? A. Bank Share capital Share premium B. Bank...
-
By finding appropriate half-cell reactions, calculate the equilibrium constant at 298.15 K for the following reactions: a. 4NiOOH(s) + 2 2 O(l) 4Ni(OH) 2 (s) + O 2 (g) b. 4NO 3 (aq)+ 4H + (aq)...
-
The cell potential E for the cell Pt(s)|H 2 (g, a H2 = 1) H + (aq, a H+ = 1)NaCl(aq, m = 0.300) AgCl(s) Ag(s) is +0.260 V. Determine Cl assuming that = Na+ = Cl .
-
The Edison storage cell is described by Fe(s) FeO(s) KOH(aq, a KOH ) Ni 2 O 3 (s) NiO(s) Ni(s) and the half-cell reactions are as follows: Ni 2 O 3 (s) + H 2 O(l) + 2e 2NiO(s) + 2OH ...
-
For anOld Country Links, Incorporated, produces sausages in three production departments Mixing , Casing and Curing, and Packaging. In the Mixing Department, meats are prepared, ground and mixed with...
-
A manufacturing firm uses a predetermined manufacturing overhead rate to allocate overhead to individual jobs, based on machine hours required. At the beginning of 2 0 1 9 , the firm expected to...
-
An investor wants to purchase a zero coupon bond from Timberlake Industries today. The bond will mature in exactly 5.00 years with a redemption value of $1,000. The investor wants a 12.00% annual...

Study smarter with the SolutionInn App


