Redo the previous problem for a large stationary Brayton cycle where the low-T heat rejection is used
Question:
Redo the previous problem for a large stationary Brayton cycle where the low-T heat rejection is used in a process application and thus has nonzero exergy.
Data from previous problem
The conversion efficiency of the Brayton cycle in Eq. 10.1 was determined with cold air properties. Find a similar formula for the second-law efficiency, assuming the low-T heat rejection is assigned a zero exergy value.
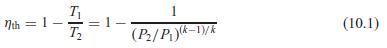
Transcribed Image Text:
T Nth = 1- T2 1 (10.1) (P/Pk-D/K
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 83% (6 reviews)
In order to redo the previous problem for a large stationary Brayton cycle with nonzero exergy for the lowT heat rejection we need to consider the exe...View the full answer

Answered By

BillClinton Muguai
I have been a tutor for the past 5 years. I have experience working with students in a variety of subject areas, including computer science, math, science, English, and history. I have also worked with students of all ages, from elementary school to college. In addition to my tutoring experience, I have a degree in education from a top university. This has given me a strong foundation in child development and learning theories, which I use to inform my tutoring practices.
I am patient and adaptable, and I work to create a positive and supportive learning environment for my students. I believe that all students have the ability to succeed, and it is my job to help them find and develop their strengths. I am confident in my ability to tutor students and help them achieve their academic goals.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Fundamentals Of Thermodynamics
ISBN: 9781118131992
8th Edition
Authors: Claus Borgnakke, Richard E. Sonntag
Question Posted:
Students also viewed these Sciences questions
-
A large stationary Brayton cycle gas turbine power plant deliver output of 100 MW to an electric generator, minimum temperature in the cycle is 300 K and maximum temperature in the cycle is 1600 K,...
-
Find the power output and the low T heat rejection rate for a Carnot cycle heat engine that receives 6 kW at 250oC and rejects heat at 30oC as in Problem 7.35.
-
A large stationary Brayton cycle gas-turbine power plant delivers a power output of 100 MW to an electric generator. The minimum temperature in the cycle is 300 K, and the maximum temperature is 1600...
-
A portfolio manager owns a bond worth 2,000,000 that will mature in one year. The pound is currently worth $1.65, and the one-year future price is $1.61. If the value of the pound were to fall, the...
-
What are the IBFs? Why did the Federal Reserve authorize the establishment of the IBFs? Explain.
-
Find the net force that the southern hemisphere of a uniformly charged sphere exerts on the northern hemisphere. Express your answer in terms of the radius R and the total charge Q.
-
3. Write a personal development objective for yourself. Make sure it meets the criteria for effective objectives shown in Figure 6.1. Show your objective to a friend or classmate, and discuss with...
-
Swit, Inc.s comparative balance sheet at January 31, 2013, and 2012, reports the following (in millions): Requirements Three situations about Swits issuance of stock and payment of dividends during...
-
please answer correctly in full and utlize accounts provided stay organizaed in return i will gove thumbs up thank you Diane Buswell is preparing the 2020 budget for one of Current Designs'...
-
The 20-year Treasury rate is 4.67 percent, and a firms credit rating is BB. Suppose management of the firm decides to raise $20 million by selling 20-year bonds. Management determines that since it...
-
A gasoline engine has a volumetric compression ratio of 9. The state before compression is 290 K, 90 kPa, and the peak cycle temperature is 1800 K. Find the pressure after expansion, the cycle net...
-
The conversion efficiency of the Brayton cycle in Eq. 10.1 was determined with cold air properties. Find a similar formula for the second-law efficiency, assuming the low-T heat rejection is assigned...
-
The business purchased on credit: a a new car for a sales representative on 1 February 2022 $36 300 ($33 000 + $3300 GST) with a depreciation rate of 20% p.a. and a residual value of $3300 ($3000 +...
-
(ii) State Wilkie's updating equation in respect of the force of inflation and explain carefully what each of the components of the equation represents. State also which type of time series process...
-
Compute the double integral D x y dA over the domain D indicated as 0 x 5, x y 2x + 3. (Use symbolic notation and fractions where needed.) f(x, y) A = D
-
4. (10 points) A researcher believes that length of time spent listening to classical music increases memory for previously learned material. She has 4 groups of 5 subjects listen to either 10 min.,...
-
We find a binary system consisting of a 1 solar mass star, still in its main sequence phase, and a white dwarf. Assume both stars formed at the same time and that they did not significantly influence...
-
The equity sections from Atticus Group's 2015 and 2016 year-end balance sheets follow. Stockholders Equity (December 31, 2015) Common stock $6 par value, 50,000 shares authorized, 35,000 shares...
-
Use the data for Lake City Industries, Inc., from P4-50B. Requirements 1. Calculate the earnings per share for Lake City Industries for the year. Assume an average of 25,000 common shares were...
-
The purpose of this case is to come up with a contingency plan[s] in order to sustain the program Move With Me, a program that serves thousands of community members throughout Lower Manhattan. The...
-
Identify the reagents necessary to produce each of the following compounds via an aldol reaction. (a) (b) (c) (d) (e)
-
Using formaldehyde and acetaldehyde as your only sources of carbon atoms, show how you could make each of the following compounds. You may find it helpful to review acetal formation. (a) (b) (c) (d)...
-
Draw a mechanism for the following transformation: NaOH, heat
-
QUESTION 3 A business owns seven flats rented out to staff at R500 per month. All flats were tenanted Ist january 21 months rent was in arrears and as at 31st December 14 months' rent wa Identify the...
-
1. 2. 3. Select the Tables sheet, select cells A6:B10, and create range names using the Create from Selection button [Formulas tab, Defined Names group]. Select cells B1:F2 and click the Name box....
-
Tropical Rainwear issues 3,000 shares of its $18 par value preferred stock for cash at $20 per share. Record the issuance of the preferred shares. (If no entry is required for a particular...

Study smarter with the SolutionInn App


