Using formaldehyde and acetaldehyde as your only sources of carbon atoms, show how you could make each
Question:
(a)
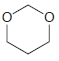
(b)
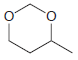
(c)
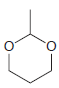
(d)
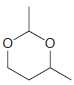
Transcribed Image Text:
O.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 63% (19 reviews)
a b c d 1 ...View the full answer

Answered By

Keziah Thiga
I am a self motivated financial professional knowledgeable in; preparation of financial reports, reconciling and managing accounts, maintaining cash flows, budgets, among other financial reports. I possess strong analytical skills with high attention to detail and accuracy. I am able to act quickly and effectively when dealing with challenging situations. I have the ability to form positive relationships with colleagues and I believe that team work is great key to performance. I always deliver quality, detailed, original (0% plagirism), well-researched and critically analyzed papers.
4.90+
1504+ Reviews
2898+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Using toluene and acetylene as your only sources of carbon atoms, show how you would prepare the following compound.
-
Using acetylene and ethylene oxide as your only sources of carbon atoms, propose a synthesis for each of the following compounds. a. b.
-
Using acetylene and methyl bromide as your only sources of carbon atoms, propose a synthesis for each of the following compounds: (a) (b) En Et Me En Et Me
-
The accompanying data are consistent with summary statistics that appeared in the paper Shape of Glass and Amount of Alcohol Poured: Comparative Study of Effect of Practice and Concentration (...
-
Domremy Drywall Inc. needs to purchase new equipment to better service its client accounts. The cost of the equipment is $175,000. It is estimated that the firm will save $35,000 annually (after tax)...
-
Write business letter to the National Tourism Organization - Seoul Office to inform them about your delegation's upcoming arrival to deliver a face-to-face presentation about the CNMI tourism...
-
If interest rates rise, a bonds current yield __________.
-
Wallace Corporation summarizes the following information from its weekly payroll records during April. Prepare the two journal entries to record the payment of the payroll and the accrual of its...
-
The current period statement of cash flows includes the following: Cash balance at the beginning of the period $450,351 Net cash flow from operating activities 164,886 Net cash flow used for...
-
QED Electronics Company had the following transactions during April while conducting its television and stereo repair business. 1. A new repair truck was purchased for $ 19,000. 2. Parts with a cost...
-
Identify the reagents necessary to produce each of the following compounds via an aldol reaction. (a) (b) (c) (d) (e)
-
Draw a mechanism for the following transformation: NaOH, heat
-
Pennington Company has the following internal control procedures over cash disbursements. Identify the internal control principle that is applicable to each procedure. 1. Company checks are...
-
Find derivative of arcsin x
-
1. Examine the impact of feedwater heater pressure on cycle efficiency and determine the intermediate pressure for optimal performance for one heater.
-
3. Environmental impact of adding feedwater heaters
-
1. Discuss the importance of genetic diversity in fish populations. 2. a) Habitat restoration and connectivity in population conservation is faced with many challenges. Discuss (5 marks) b)a) ...
-
1. Assumethatlengthin Oreochromis variabilis (Victoria Tilapia) ispolygenicvaryingfrom30cmto50cm. A 30cm purebred parent is crossed with another purebred 50 cm individual and the resulting F 1...
-
In Exercises 55 through 60, find the second derivative of the given function. h(t) = (t 2 + 5) 8
-
You are standing at x = 9.0 km and your assistant is standing at x = 3.0 km. Lightning bolt 1 strikes at x = 0 km and lightning bolt 2 strikes at x = 12.0 km. You see the flash from bolt 2 at t = 10...
-
Compound A, C6H12O2, was found to be optically active, and it was slowly oxidized to an optically active carboxylic acid B, C6H12O3, by +Ag(NH3)2. Oxidation of A by anhydrous CrO3 gave an optically...
-
Remembering that a protonated aldehyde or ketone is a type of carbocation, and that carbocations are electrophiles, propose a curved-arrow mechanism for the electrophilic aromatic substitution...
-
From your knowledge of the reactivity of LiAlH4, as well as the reactivity of epoxides with nucleophiles, predict the product (including stereochemistry, if appropriate) in each of the following...
-
An underlying asset price is at 100, its annual volatility is 25% and the risk free interest rate is 5%. A European call option has a strike of 85 and a maturity of 40 days. Its BlackScholes price is...
-
Prescott Football Manufacturing had the following operating results for 2 0 1 9 : sales = $ 3 0 , 8 2 4 ; cost of goods sold = $ 2 1 , 9 7 4 ; depreciation expense = $ 3 , 6 0 3 ; interest expense =...
-
On January 1, 2018, Brooks Corporation exchanged $1,259,000 fair-value consideration for all of the outstanding voting stock of Chandler, Inc. At the acquisition date, Chandler had a book value equal...

Study smarter with the SolutionInn App


