Using acetylene and methyl bromide as your only sources of carbon atoms, propose a synthesis for each
Question:
(a)
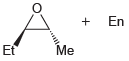
(b)
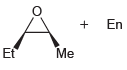
Transcribed Image Text:
En Et Me En Et Me
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 57% (14 reviews)
a b 1 H Lindlars ca...View the full answer

Answered By

Felix Mucee
I am a detailed and thorough professional writer with 5 years of administrative experience- the last 2 years in academic writing and virtual office environment. I specialize in delivering quality services with respect to strict deadlines and high expectations. I am equipped with a dedicated home office complete with a computer, copier/scanner/fax and color printer.
I provide creative and detailed administrative, web search, academic writing, data entry, Personal assistant, Content writing, Translation, Academic writing, editing and proofreading services. I excel at working under tight deadlines with strict expectations. I possess the self-discipline and time management skills necessary to have served as an academic writer for the past five years. I can bring value to your business and help solve your administrative assistant issues.
4.70+
13+ Reviews
33+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Dimethoxyethane (DME) is a polar aprotic solvent often used for S N 2 reactions. Propose a plausible synthesis for DME using acetylene and methyl iodide as your only sources of carbon atoms....
-
Using acetylene and 2-methylpropane as your only sources of carbon atoms, propose a plausible synthesis for 4-methyl-2-pentanone. You will need to utilize many reactions from previous chapters.
-
Using acetylene and ethylene oxide as your only sources of carbon atoms, propose a synthesis for each of the following compounds. a. b.
-
Return to Better Mousetraps in Problem 18. Suppose the firm can cut its requirements for working capital in half by using better inventory control systems. By how much will this increase project NPV?...
-
What would be the advantage to an exporter of buying a futures contract on the foreign exchange rate value of the dollar?
-
Katrina has wages of $150,000 in 2022. She also operates a small business that generated net profits of $10,000. What is Katrinas total self-employment tax? a. $0 b. $267.82 c. $290.00 d. $1,530.00
-
A representative is selected at random. Find the probability of each event. (a) The representative is male. (b) The representative is a Republican. (c) The representative is male given that the...
-
Casey Carpet manufactures broadloom carpet in seven processes: spinning, dyeing, plying, spooling, tufting, latexing, and shearing. In the Dyeing Department, direct materials (dye) are added at the...
-
Which of the following statement about the selection of allocation base is INCORRECT? A) The default base should always be used. B) A company should strive to select the allocation base that is the...
-
Given a string, reduce it in such a way that all of its substrings are distinct. To do so, you may delete any characters at any index. What is the minimum number of deletions needed? Note: A...
-
A bond that pays interest forever and has no maturity date is a perpetual bond. How is the yield to maturity on such a bond determined?
-
Draw a mechanism and predict the major product for each reaction. a. b. c. d. e. f. ? 1) LAH H. 2) H20
-
The length of the longest ladder that can negotiate the corner depicted in Fig. P15.17 can be determined by computing the value of u that minimizes the following function: For the case where w 1 = w...
-
The accounting records of the Eco Paper Company include the following information relating to the current year ended 31 March 2023: Materials 31 March 2023 $20,000 1 April 2022 $25,000 Work in...
-
The first read is an article on the development of money of a World War II prisoner-of-war, which was published in 1945. The second article was published in the opinion section of the New York Times...
-
Describe each Speaker's basic assumptions regarding employee motivation. That is, what are the underlying principles which guide how the Speaker treats his/her people (i.e., their direct report...
-
Find the area of the shaded region. The graph to the rate of IQ scores of adults, and those scores are normally distributed with the mean of 100 and a standard deviation of 15. x=81
-
In which scenario is Nikki showing resilience to stress? Nikki lost her job as an engineer 3 months ago. At first, she was depressed, but she realized she wanted to change career paths and decided to...
-
Combine the expressions by writing them as a logarithm of a single expression. log645 + logb
-
1. Firms may hold financial assets to earn returns. How the firm would classify financial assets? What treatment will such financial assets get in the financial statements in accordance with US GAAP...
-
Identify the reagents a?f in the following scheme: .Br CH-
-
Galactose, a constituent of the disaccharide lactose found in dairy products, is metabolized by a pathway that includes the isomerization of UDP-galactose to UDP-glucose, where UDP = uridylyl...
-
Propose a structure consistent with the following spectral data for a compound C8H18O2: IR: 3350 cm1. 1H NMR: 1.24 (12 H, singlet); 1.56 (4 H, singlet); 1.95 (2 H, singlet)
-
Which of the following programs covers custodial care? A HMOs B Medicare Part B C PPOs D Medicare Part A E Medicaid
-
uppose a taxpayer has exhausted his lifetime exclusion amount and has $14 million. a. Assuming a flat 40% gift tax rate, what is the maximum amount a taxpayer can transfer to her daughter (and still...
-
Physical Units Method, Relative Sales Value Method Farleigh Petroleum, Inc., is a small company that acquires high - grade crude oil from low - volume production wells owned by individuals and small...

Study smarter with the SolutionInn App


