Show that the van der Waals equation can be written as a cubic equation in the compressibility
Question:
Show that the van der Waals equation can be written as a cubic equation in the compressibility factor, as in Eq. 12.53.
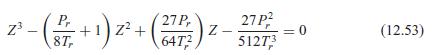
Transcribed Image Text:
2 () () - 27P, +1) z+ 6472 27P? 51273 z - = 0 (12.53)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 58% (12 reviews)
The van der Waals equation of state is given by P aV2 V b RT where P is the pressure V is the molar ...View the full answer

Answered By

User l_917591
As a Business Management graduate from Moi University, I had the opportunity to work as a tutor for undergraduate students in the same field. This experience allowed me to apply the theoretical knowledge I had gained in a practical setting, while also honing my teaching and communication skills.
As a tutor, I was responsible for conducting tutorial sessions, grading assignments and exams, and providing feedback and support to my students. I also assisted with the preparation of course materials and collaborated with other tutors and professors to ensure consistency in teaching and assessment.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Fundamentals Of Thermodynamics
ISBN: 9781118131992
8th Edition
Authors: Claus Borgnakke, Richard E. Sonntag
Question Posted:
Students also viewed these Sciences questions
-
Show that van der Waals equation can be written as a cubic equation in the compressibility factor involving the reduced pressure and reduced temperature as (27 P 27 P2 512 T, (T+1) z2- - 1) z - Z3 ST...
-
Show that the van der Waals equation of state does not satisfy the criteria of intrinsic stability for all values of the parameters. Sketch the curves of P versus V for constant T (the isotherms of...
-
The van der Waals equation of state, an approximate representation of the behavior of gases at high pressure, is given by Where a and b are constants having different values for different gases. (In...
-
Parent Ltd owns 80% of Subsidiary Ltd. In the financial year ended 30 June 20X2, Subsidiary Ltd sold inventory to Parent Ltd. Details regarding the transaction are as follows: Cost to Subsidiary to...
-
What type of exchange rate system was the gold standard? Explain how it operated?
-
(a) Shallow water is non dispersive; the waves travel at a speed that is proportional to the square root of the depth. In deep water, however, the waves can't "feel" all the way down to the...
-
1. How can you evaluate whether this training was successful? If possible, try to conduct an evaluation of what the class learned. What do the results of this evaluation indicate?
-
A stream of air enters a 7.50-cm ID pipe at a velocity of 60.0 m/s at 27C and 1.80 bar (gauge). At a point downstream, the air flows through a 5.00 cm ID pipe at 60C and 1.53bar (gauge). What is the...
-
16. Ron Corporation had the following selected account balances and fair values on December 31, 2020 when it was acquired by Valley Company. Book Values Fair Values Receivables $ 55,000 $ 55,000...
-
Many regions along the coast in North and South Carolina and Georgia have experienced rapid population growth over the last 10 years. It is expected that the growth will continue over the next 10...
-
Show how to find the constants in Eq. 12.52 for the van der Waals EOS. Vc = 3b 27 R?T? a 64 P. (12.52) RT. b 8P.
-
Find the heat of evaporation, hfg, for R-134a at 0C from the generalized charts and compare to the value in Table B.5.
-
The government can finance its spending by __________________,__________________ ,or __________________.
-
Consider the expression timing is everything in relation to the building of the TOMS brand. Besides the influence of recovering economic conditions and the increased affluence of potential customers,...
-
What is corporate strategy and why is it important? Choose a company with which you are familiar, and evaluate its corporate strategy, especially in regards to financial strategies. What are some...
-
Assignment Tasks: Review the following situations and for each pay period determine the employee's net pay by calculating what earnings & benefits are subject to Income Tax, Canada / Quebec Pension...
-
sample letter for signature change on bank accounts for principals of school
-
Use Excelshowing all work and formulasto complete the following: Prepare a flexible budget. Compute the sales volume variance and the variable cost volume variances based on a comparison between...
-
Determine whether the following bonds payable will be issued at par, at a premium, or at a discount: a. The market interest rate is 5 percent. Wilson Corp. issues bonds payable with a stated rate of...
-
Is the modified 5-question approach to ethical decision making superior to the modified moral standards or modified Past in approach?
-
For a pair of keto-enol tautomers, explain how IR spectroscopy might be used to identify whether the equilibrium favors the ketone or the enol.
-
Acrolein is an α,β-unsaturated aldehyde that is used in the production of a variety of polymers. Acrolein can be prepared by treating glycerol with an acid catalyst. Propose...
-
Draw the structure of the product with molecular formula C 10 H 10 O that is obtained when the compound below is heated with aqueous acid. CN CN C10H100 Heat
-
Diplomatic Security Service provides Airport Transportation and Surveillance Service to Foreign Diplomats in Guyana. The company has two support departments - Information Systems and Equipment...
-
Q1: A disparity of bargaining power between the parties to a contract may result in unfair terms but a court is not likely to consider the contract unconscionable. Group of answer choices a. True b....
-
Life Tool Manufacturing has a system in place to recall products that prove to be dangerous at some time after manufacture and distribution. This represents which element of the due care theory?...

Study smarter with the SolutionInn App


