Each of the following compounds exists as a fluxional molecule that is interconverted into one or more
Question:
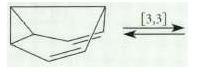
Transcribed Image Text:
[3,3]
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 83% (6 reviews)
The s bond that moves is indica...View the full answer

Answered By

Mary Boke
As an online tutor with over seven years of experience and a PhD in Education, I have had the opportunity to work with a wide range of students from diverse backgrounds. My experience in education has allowed me to develop a deep understanding of how students learn and the various approaches that can be used to facilitate their learning. I believe in creating a positive and inclusive learning environment that encourages students to ask questions and engage with the material. I work closely with my students to understand their individual learning styles, strengths, and challenges to tailor my approach accordingly. I also place a strong emphasis on building strong relationships with my students, which fosters trust and creates a supportive learning environment. Overall, my goal as an online tutor is to help students achieve their academic goals and develop a lifelong love of learning. I believe that education is a transformative experience that has the power to change lives, and I am committed to helping my students realize their full potential.
5.00+
4+ Reviews
21+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Each of the following compounds exhibits two electrophilic centers. Identify both centers in each compound. (You will need to draw resonance structures in each case.) a. b. c. H. A cockroach...
-
Each of the following compounds has been prepared from o-anisidine (o-methoxyaniline). Outline a series of steps leading to each one. (a) o-Bromoanisole (d) 3-Fluoro-4-methoxybenzonitrile (b)...
-
Each of the following compounds has three possible names listed for it. For each compound, what is the correct name and why arent the other names used? a. N2O: nitrogen oxide, nitrogen (I) oxide,...
-
Describe the relationship of the AD, SRAS, and LRAS curves when the economy is in long-run macroeconomic equilibrium.
-
Ross and MIC produce 2 different cars. Each firm can be aggressive in advertising and marketing. If only one of the 2 firms choose high advertising the NPV of that firm would be $200M whereas the NPV...
-
Name four CIA methods that can be utilised.
-
Extinct New Zealand birds. Refer to the Evolutionary Ecology Research (July 2003) study of extinction in the New Zealand bird population, presented in Exercise 1.20 (p. 49). Recall that biologists...
-
X. R. Lopez and L. O. Moore, interior designers, are forming a partnership. Both plan to work in the firm on a full-time basis. Lopezs initial investment is $ 20,000; Moores investment, $ 30,000....
-
its only highschool Mark's Repair Shop-Balance Sheet and Income Statement Balance Sheet Mark's Repair Shop Balance Sheet September 30, 20- Liabilities 500 Accounts Payable Assets Cash Accounts...
-
You are a senior accountant in a top-tier accounting firm. Your senior manager has asked you to assist your client, Daisy Ltd, in the preparation of consolidated financial statements for the year...
-
Show how the transition state for a [3,3] sigmatropic reaction can be analyzed as the interaction of two ally lie radicals, and that the same stereochemical outcome is predicted.
-
When previtamin D2 is heated, two compounds, A and B, are obtained that are stereoisomers of both ergosterol and lumisteroL Suggest structures for these compounds, and explain mechanistically how...
-
The vertical surface of a reservoir dam that is in contact with the water is 120 m wide and 12 m high. The air pressure is one atmosphere. Find the magnitude of the total force acting on this surface...
-
Watch the clip from HBO's Chernobyl and respond to the questions below using complete sentences....
-
1) Every organization has a unique culture. As you move forward with a travel or per diem position, what steps will you take to learn about your assignment organization's culture? 2)Flexibility and...
-
See the right figure of the lifting and transporting equipment. During operation, while a mass of 1.5 x 104 kg was descending at a constant speed v = 15 m/min, the machine experienced a breakdown,...
-
PROBLEM 3 BABEY Company makes three products with the following characteristics: Product V jk jin Selling price per unit 10 15 20 Variable cost per unit 6 10 10 Machine hours per unit 2 4 10 The...
-
Diversified Semiconductors sells perishable electronic components. Some must be shipped and stored in reusable protective containers. Customers pay a deposit for each container received. The deposit...
-
(a) Write the equation for perchloric acid (HClO 4 ) dissolving in water. (b) Write the equation for sodium nitrate dissolving in water.
-
An item of depreciable machinery was acquired on 1 July 2009 for $120,000 by cash It is expected to have a useful life of 10 years and zero salvage value On 1 July 2012, it was decided to revalue the...
-
Draw structures corresponding to the following IUPAC names: (a) 1, 1-Dimethylcycloocatne (b) 2-Cyclobutylhexane (c) 1, 2-Dichlorocyclopentane (d) 1, 3-Dibromo-5-methylcyclohexane
-
Name the following cycloalkanes:
-
Name the following substances, including the cis- or trans-prefix: CH2CH3 (b) , (a) H. - - CI
-
The Regal Cycle Company manufactures three types of bicyclesa dirt bike, a mountain bike, and a racing bike. Data on sales and expenses for the past quarter follow: Total Dirt Bikes Mountain Bikes...
-
?? A local college is deciding whether to conduct a campus beautification initiative that would imvolve various projects, such as planting trees and remodeling bulidings, to make the campus more...
-
A company has net income of $196,000, a profit margin of 9.7 percent, and an accounts receivable balance of $135,370. Assuming 70 percent of sales are on credit, what is the companys days sales in...

Study smarter with the SolutionInn App


