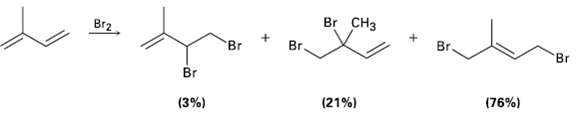
Electrophilic addition of Br2 to isoprene (2-methyl-1, 3-hutadiene) yields the following product mixture: Of the 1, 2-addition
Question:
Electrophilic addition of Br2 to isoprene (2-methyl-1, 3-hutadiene) yields the following product mixture: Of the 1, 2-addition products, explain why 3, 4-dibromo-3-methyl-1-butene (21 %) predominates over 3, 4-dibromo-2-mcthyl-1-butene(3%).
Transcribed Image Text:
Br CHз Br2 Br. Br Br 'Br Br (76%) (21%) (3%)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 90% (10 reviews)
CH3 8 8 BrCH CCHCH A tertiaryprimary allylic carboca...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
The rate law for addition of Br2 to an alkene is first order in Br2 and first order in the alkene. Does this fact prove that the mechanism of addition of Br2 to an alkene proceeds in the same manner...
-
Explain why methyl propyl ether forms both methyl iodide and propyl iodide when it is heated with excess HI.
-
Explain why the following reaction yields the Hofmann product exclusively (no Zaitsev product at all) even though the base is not sterically hindered: Br NaOEt ELOH
-
Recall the heat equation which we solved numerically T= = DTxx There we implemented an explicit numerical scheme (FTCS) which led to a conditionally stable solution - meaning that for certain time...
-
Would an employee who posts, "I hate my job!" be protected, according to the principles cited here? Why or why not?
-
Following is the consolidated statement of stockholders equity of Costco Wholesale Corporation for the year ended August 28, 2011: Required 1. Costco has an item in the statement of stockholders...
-
Define communication.
-
What are some pros and cons of holding high levels of current assets in relation to sales? Use the DuPont equation to help explain your answer.
-
Drs. Glenn Feltham and David Ambrose began operations of their physical therapy clinic, called Northland Physical Therapy, on January 1, 2017. The annual reporting period ends December 31. The trial...
-
Using the 4-quarter moving average: What is the forecast for 3Q 2022, 4Q 2022, 1Q 2023, and 2Q 2023 2. Compute the forecast for 3Q 2022, 4Q 2022, 1Q 2023, and 2Q 2023 using exponential smoothing...
-
Treatment of 3, 4-dibromohexane with strong base leads to loss of 2 equivalents of HBr and formation of a product with formula C6H10. Three products are possible. Name each of the three, and tell how...
-
Propose a structure for a conjugated diene that gives the same product from both 1, 2- and 1, 4-addition of HBr.
-
The tenant agrees to lease the premises for 12 months beginning on September 1 and at a monthly rental charge of $800. Let t: You are a tenant. m: You lease the premises for 12 months. s: You will...
-
Outlines help in several ways: They help organize your thoughts so your speech is easy to follow. They keep you on track so you don't research beyond the scope of your speech. They give you a clear...
-
As a nurse leader you have to be able to have the willingness to be in a place of flexibility for change. Sometimes change can cause stress that can cloud our thoughts and ability to connect. Share a...
-
find T(625). I Given the recurrence relation T(n)=7T (n/5)+ 10n for n > 1 T (1)=1 Answer: (please write your answer here, add required space if needed)
-
2. (10 pts) The following program has many compilation errors. Underline each of the compilation errors, then rewrite each statement (even the correct ones) so that all these errors are fixed. Do not...
-
In this problem you will implement a variant of the List ADT. In particular you will implement the String-List ADT, in a concrete class called SListArray, based on the provided abstract Slist class....
-
How can frequency distributions help us to better understand our data?
-
Could a set of three vectors in span all of? Explain. What about n vectors in when n is less than m? R4
-
The enthalpy of solution for NaOH is -44.46 kJ/mol. What can you conclude about the relative magnitudes of the absolute values of Hsolute and Hhydration , where Hsolute is the heat associated with...
-
Predict the major products of the following reactions. (a) 2, 4-dinitrochlorobenzene + NaOCH3 (b) Phenol + tert-butyl chloride + AlCl3 (c) Nitrobenzene + fuming sulfuric acid (d) Nitrobenzene +...
-
Predict the major products of bromination of the following compounds, using Br2 and FeBr3 in the dark. (a) (b) (c) NO, OCH OCH3 OCH,
-
What products would you expect from the following coupling reactions? (a) (b) (c) (d) (e) Br 2 CuLi PdCl2 Pd catalyst, base OR OR Pd catalyst, base Pd OAc)2 PPh;
-
Accounting changes fall into one of three categories. Identify and explain these categories and give an example of each one.
-
Machinery is purchased on May 15, 2015 for $120,000 with a $10,000 salvage value and a five year life. The half year convention is followed. What method of depreciation will give the highest amount...
-
Flint Corporation was organized on January 1, 2020. It is authorized to issue 14,000 shares of 8%, $100 par value preferred stock, and 514,000 shares of no-par common stock with a stated value of $2...

Study smarter with the SolutionInn App


