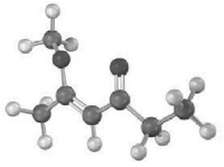
Estimate the chemical shift of each carbon in the following molecule. Predict which carbons will appear in
Question:
Estimate the chemical shift of each carbon in the following molecule. Predict which carbons will appear in the DWPT-90 spectrum, which will give positive peaks in the DEPT-135 spectrum, and which will give negative peaks in the DEPT-135spectrum.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (9 reviews)
Strategy Identify the carbons as CH3 CH2 CH or quaternary and use Figure 137 to ...View the full answer

Answered By

Shem Ongek
I am a professional who has the highest levels of self-motivation. Additionally, I am always angled at ensuring that my clients get the best of the quality work possible within the deadline. Additionally, I write high quality business papers, generate quality feedback with more focus being on the accounting analysis. I additionally have helped various students here in the past with their research papers which made them move from the C grade to an A-grade. You can trust me 100% with your work and for sure I will handle your papers as if it were my assignment. That is the kind of professionalism that I swore to operate within. I think when rating the quality of my work, 98% of the students I work for always come back with more work which therefore makes me to be just the right person to handle your paper.
4.80+
174+ Reviews
426+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Give the hybridization state of each carbon in the following compounds: (b) Formaldehyde (H2C==O) (c) Ketene (H2C==C==O) (d) Propene (CH3CH==CH2) (e) Acetone [(CH3)2C==O] (f) Acrylonitrile (CH2==CHCN)
-
The chemical shift of the CH3 protons in diethyl ether is, = 1.16 and that of the CH2 protons is 3.36. What is the difference in local magnetic field between the two regions of the molecule when the...
-
Explain why the chemical shift of the OH proton of a carboxylic acid is at a higher frequency than the chemical shift of an OH proton of an alcohol.
-
What risks accompany a standby credit letter for (a) the issuer and (b) the beneficiary?
-
The following are the approximate market shares of different brands of soft drinks during the 1980s: Coke-40% Pepsi-30% 7-Up-10% Dr. Pepper-10% All other brands-10% a. Compute the Herfindahl for the...
-
What is the force F on the 1.0 nC charge in Figure EX22.19? Give your answer as a magnitude and a direction. 1.0 nC 1.0 cm ,' 1.0 cm 60 60% 1.0 cm 2.0 nC 2.0 nC FIGURE EX22.19
-
Consider the following partially completed one-way ANOVA summary table: Source Sum of Squares Degrees of Freedom Mean Sum of Squares F Between 4 Within 60 Total 76 24 a. Complete the remaining...
-
Returns and Standard Deviations consider the following information: a. What is the expected return on an equally weighted portfolio of these three stocks? b. What is the variance of a portfolio...
-
1/. List and describe three payroll services that must be provided by a registered BAS agent. 2/. List and describe three business services that do not require registration of BAS Agent. (Please...
-
GuestLodge, Inc. also has the right to operate a golf course near the national park. GuestLodge sells punch cards to the course, where customers can pay $500 to purchase a "10-Pack" (10 rounds of...
-
Assign a chemical shift to each carbon in 6.methyl-5-hepten-2-ol(figure). (a) OH 0 ppm 200 180 160 140 120 100 80 60 40 20 Chemical shift (8) (b) 200 180 120 160 100 60 140 80 0 ppm Chemical shift...
-
Propose a structure for an aromatic hydrocarbon, C 11 H 16 that has the following 13 C NMR spectral data: Broadband-decoupled 13 C NMR: 29.5, 31.8, 50.2, 125.5, 127.5, 130.3, 139.8 DEPT-90: 125.5,...
-
Mark wants to purchase a new luxury sedan for $50,000 for use exclusively in his business. The car salesman told Mark that he could expense $25,000 of the cost immediately using Section 179 and...
-
1. Identify an industry that competes internationally (i.e., fast food, clothing, sportswear, automotive, etc). All your companies must be from ONE Industry (you cannot discuss Taco Bell and Nike)....
-
A research article on " Leadership in Project Management: Cultivating Strong Employee-Employer Bonds" shows major findings on why big companies fail in leadership skill practice. How they can...
-
Discuss and Identify the current types of stock, such as common or preferred stock, currently issued, and outstanding. Include a narrative description along with the values and number of shares found...
-
The organization we intend to study is Local Point, a student cafeteria run by UW Housing & Food Services. Our team would like to figure out how to utilize modern technology and rational...
-
Briefly summarize the Coase Theorem (include the 3 key conditions). List the major types of approaches government typically takes to deal with negative externalities. Suppose the demand for...
-
How would the planning team interpret the mean values for the variables?
-
Compile data on consumption and expenditures for the following categories in 30 different countries: (1) food and beverages, (2) clothing and footwear, (3) housing and home operations, (4) household...
-
What mass of salt (NaCl) should you add to 1.00 L of water in an ice-cream maker to make a solution that freezes at -10.0 C? Assume complete dissociation of the NaCl and density of 1.00 g/mL for...
-
Predict the products you expect when the following starting material undergoes oxidation with an excess of each of the reagents shown below. (a) Chromic acid (b) PCC (Pyridinium Chlorochromate) (c)...
-
Show how you would use simple chemical tests to distinguish between the following pairs of compounds. In each case, describe what you would do and what you would observe. (a) Butan-1-ol and...
-
Write the important resonance forms of the following anions. (a) (b) (c) 1 O-S-CH
-
Suppose the S&P 500 currently has a level of 960. One contract of S&P 500 index futures has a size of $250 S&P 500 index. You wish to hedge an $800,000-portfolio that has a beta of 1.2. (A)In order...
-
Exhibit 4.1 The balance sheet and income statement shown below are for Koski Inc. Note that the firm has no amortization charges, it does not lease any assets, none of its debt must be retired during...
-
Haley is 57 years of age. She is planning for future long-term care needs. She knows that yearly nursing home costs in her area are currently $69,000, with prices increased by 5 percent annually....
Introduction To Continuum Mechanics For Engineers 1st Revised Edition - ISBN: 0486474607 - Free Book

Study smarter with the SolutionInn App


