Explain whether the electron arrangement for these atoms is the ground state or an excitedstate: a) Energy
Question:
Explain whether the electron arrangement for these atoms is the ground state or an excitedstate:
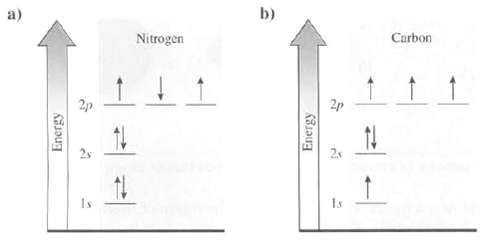
Transcribed Image Text:
a) Energy 3 Nitrogen b) Energy 2p 5 4 ↑ Carbon
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (12 reviews)
The electron arrangement that is in accord with the basic rul...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
An electron is in the ground state in a two-dimensional, square, infinite potential well with edge lengths L. We will probe for it in a square of area 400 pm2 that is centered at x = L/8 and y = L/8....
-
For an electron in the ground state of a hydrogen atom, calculate its (a) Potential energy, (b) Kinetic energy, and (c) Total energy.
-
An electron is in the ground state in a two-dimensional, square, infinite potential well with edge lengths L. We will probe for it in a square of area 400 pm2 that is centered at x = L/8 and y = L/8....
-
It is stated in Section 40.3 that a finite potential well always has at least one bound level, no matter how shallow the well. Does this mean that as U 0 0, E 1 0? Does this violate the Heisenberg...
-
Rabbid Industries Ltd consists of three decentralized divisions: Brentwood Division, Crater Division and Dollar Division. The managing director of Rabbid Industries has given the managers of the...
-
The stockholders equity section of Benton Corporations balance sheet as of December 31, 2014 is as follows: Stockholders Equity Preferred Stock, $5 par value, authorized-15000 shares, issued-4000...
-
Driver gear bore holes. During the manufacture of the driver gear of an automobile, a dial bore gauge is used to bore a hole into cast steel. Specifications require that the diameter of the bore hole...
-
International Steel Company has budgeted manufacturing overhead costs of $2.5 million. It has allocated over- head on a plant-wide basis to its two products (Standard Steel and Deluxe Steel) using...
-
The general fund of Loveland ordered a new fire truck on November 12, 20X8, for $150,000. The order was appropriately encumbered on this date. Loveland received the fire truck on January 15, 20X9,...
-
Prove that alphabeta pruning takes time O(2 m/2 ) with optimal move ordering, where m is the maximum depth of the game tree.
-
Show an atomic orbital energy level diagram for these atoms: (a) Si (b) Al (c) Cl
-
Show an energy level diagram for the MOs for He 2 and show how the electrons would be arranged in these MOs.
-
The electric field outside a charged conducting sphere is the same as if the charge were centered at its origin.Use this fact to calculate the capacitance of a sphere of radius R, taking the second...
-
1. Identify an industry that competes internationally (i.e., fast food, clothing, sportswear, automotive, etc). All your companies must be from ONE Industry (you cannot discuss Taco Bell and Nike)....
-
A research article on " Leadership in Project Management: Cultivating Strong Employee-Employer Bonds" shows major findings on why big companies fail in leadership skill practice. How they can...
-
Discuss and Identify the current types of stock, such as common or preferred stock, currently issued, and outstanding. Include a narrative description along with the values and number of shares found...
-
The organization we intend to study is Local Point, a student cafeteria run by UW Housing & Food Services. Our team would like to figure out how to utilize modern technology and rational...
-
Briefly summarize the Coase Theorem (include the 3 key conditions). List the major types of approaches government typically takes to deal with negative externalities. Suppose the demand for...
-
Describe how managers should deal with problematic behavior.
-
Subprime loans have higher loss rates than many other types of loans. Explain why lenders offer subprime loans. Describe the characteristics of the typical borrower in a subprime consumer loan.
-
What is wrong with the following calculation? 3 (-4) --- 1 --4 dx = = -1 X 1 3 3
-
Starting from testosterone (Problem 17.48), how would you prepare the followingsubstances? CH (a) (b) C CH - (c) (d) CH CH -
-
Compound A, C10H18O, undergoes reaction with dilute H2SO4 at 25 C to yield a mixture of two alkenes, C10H16. The major alkene product, B, gives only Cyclopentanone after ozone treatment followed by...
-
Dehydration of trans-2-methylcyclopentanol with POCl3 in pyridine yields predominantly 3-methylcyclopentene. Is the stereochemistry of this dehydration syn or anti? Can you suggest a reason for...
-
Mediocre Company has sales of $120,000, fixed expenses of $24,000, and a net income of $12,000. If sales rose 10%, the new net income would be: Question 18 options: $16,800 $36,000 $13,200 $15,600
-
1. Why might managers of small restaurants decide not to adopt the standard work hour approach to controlling labour cost? (minimum 150 words )
-
Which statement is true regarding the U.S. GAAP impairment test for limited life intangibles? A. U.S. GAAP impairment is likely to be greater than IFRS impairment. B. The impairment test for limited...

Study smarter with the SolutionInn App


