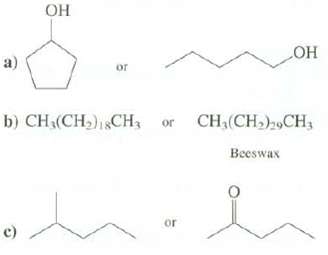
Explain which compound you expect to have the higher melting point. c) of b) CH(CH)1CH or or
Question:
Explain which compound you expect to have the higher melting point.
Transcribed Image Text:
ОН c) of b) CH₂(CH₂)1CH₁ or or ОН CH₂(CH₂)29CH, Beeswax
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 61% (18 reviews)
a The cyclic alcohol mp 19C has a higher melting point than the straight chain ...View the full answer

Answered By

Emel Khan
I have the ability to effectively communicate and demonstrate concepts to students. Through my practical application of the subject required, I am able to provide real-world examples and clarify complex ideas. This helps students to better understand and retain the information, leading to improved performance and confidence in their abilities. Additionally, my hands-on approach allows for interactive lessons and personalized instruction, catering to the individual needs and learning styles of each student.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Explain which compound you expect to have the higher boiling point. a) cont b) CH3CHCOH c) CH,CH,OCH,CH, d) CH H or or CHCHCHCH or CHCH,CH,OCH,CH,CH, or H
-
Which compound would you expect to have the higher melting point, propane or cyclopropane? Explain your answer.
-
Explain which compound has the higher melting point or boiling point: a) Melting point b) Boiling point or C COCH c) Boiling point or
-
Shemekia applied to a college that uses multiple regression to select students. This college only considers students whose predicted firstyear GPA is 3.0 or higher. Shemekias predicted GPA was 2.9...
-
Grant Lawson has just been appointed as the new financial controller of Safety Chemicals Ltd, which has three separate divisions Industrial Chemicals, Paints, and Household Chemicals. During his...
-
Zee-Drive Ltd. is a computer manufacturer. One of the items they make is monitors. Zee-Drive has the opportunity to purchase 16,400 monitors from an outside supplier for $205 per unit. One of the...
-
3 Identify the different stages through which cross-cultural negotiations tend to move. Explain how each stage influences the outcome of negotiations.
-
On January 1, 2017, Spring Fashions Inc. enters into a contract with a southeast retail company to provide 500 dresses for $ 62,500 ($ 125 per dress) over the next 10 months. On October 1, 2017,...
-
Exercise 10-2 (Algo) Direct Labor Variances [LO10-2] SkyChefs, Inc., prepares in-flight meals for a number of major airlines. One of the companys products is grilled salmon with new potatoes and...
-
1. Complete pages 1 and 2 of Form 1040 for Marc and Michelle. 2. Complete Schedule 1 of Form 1040 for Marc and Michelle. (use the most recent form available). Form 1040: Not sure if correct? Schedule...
-
Explain the difference in the melting points of these isomers: mp=-140C 0 mp = 7C
-
Which of these two salts would your expect to be more soluble in hexane(C6H14)? NH, CI + Or N(CHCHCHCH3)4 Cl
-
1. When water emerges from a faucet, the stream narrows as the water falls. Explain why. 2. Find the diameter of a tube that would give double the flow rate for the pressure difference in Problem 57.
-
If f ( x ) = ( 1 3 - In ( x ) ) ^ 8 , determine f ' ( 1 ) .
-
1. ThestocksAandBhavethefollowingdistributionsofreturns. A B Probability State1 3 4 0.2 State2 5 2 0.3 State3 4 8 0.2 State4 6 5 0.1 State5 6 1 0.2 2....
-
Define nested designs. Explain why the nested designs are important.
-
3 x y 3 + x y = l n ( x ) solve for d y d x
-
Let ln ( xy ) + y ^ 8 = x ^ 7 + 2 . Find dy / dx .
-
1 Use Figures 12.4 and 12.5 to analyse the organisations stage of development in using IS. Review Sections 12.6 to 12.8. Has the organization used one or more of these systems, and what have been...
-
Citing a scientific article, explain in your own words, how DNA fingerprinting has been used in forensic science to solve crimes and why it may not always be accurate or effective.
-
For the region S in Example 1, show that the sum of the areas of the upper approximating rectangles approaches 1/3, that is, Data from Example 1 Use rectangles to estimate the area under the parabola...
-
Methyl aryl ethers, such as anisole, are cleaved to iodomethane and a phenoxide ion by treatment with LiI in hot DMF. Propose a mechanism for this reaction.
-
Tert-Butyl ethers can be prepared by the reaction of an alcohol with 2-methyipropene in the presence of an acid catalyst. Propose a mechanism for this reaction.
-
Meerwein?s reagent, triethyloxonium tetra-fluoroborate, is a powerful ethylating agent that converts alcohols into ethyl ethers at neutral pH. Show the reaction of Meerwein?s reagent with...
-
Indicate whether the following managerial policy increases the risk of a death spiral:Use of low operating leverage for productionGroup of answer choicesTrueFalse
-
It is typically inappropriate to include the costs of excess capacity in product prices; instead, it should be written off directly to an expense account.Group of answer choicesTrueFalse
-
Firms can avoid the death spiral by excluding excess capacity from their activity bases. Group of answer choicesTrueFalse

Study smarter with the SolutionInn App


