Explain which compound is a stronger acid: a) CHCCH, or CHCCHC=N CCH3 or
Question:
Explain which compound is a stronger acid:
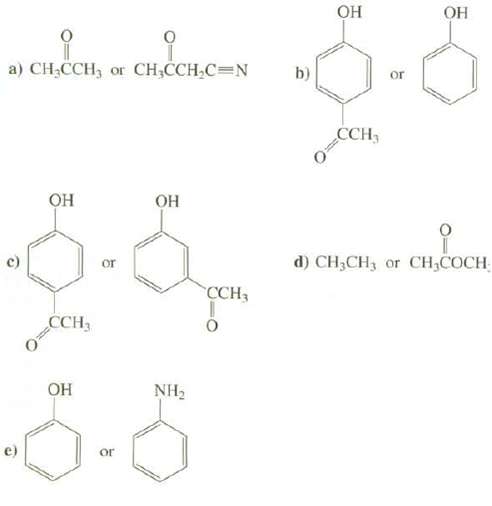
Transcribed Image Text:
a) CH₂CCH, or CH₂CCH₂C=N О З ОН CCH3 ОН or or ОН NH₂ CCH3 b) ОН CCH- or ОН сню сосн d) CH₂CH3 or CH₂COCH
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 70% (17 reviews)
a When an H is removed from the CH 2 group of the compound on the right the electron pair of the con...View the full answer

Answered By

Felix Mucee
I am a detailed and thorough professional writer with 5 years of administrative experience- the last 2 years in academic writing and virtual office environment. I specialize in delivering quality services with respect to strict deadlines and high expectations. I am equipped with a dedicated home office complete with a computer, copier/scanner/fax and color printer.
I provide creative and detailed administrative, web search, academic writing, data entry, Personal assistant, Content writing, Translation, Academic writing, editing and proofreading services. I excel at working under tight deadlines with strict expectations. I possess the self-discipline and time management skills necessary to have served as an academic writer for the past five years. I can bring value to your business and help solve your administrative assistant issues.
4.70+
13+ Reviews
33+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Explain the following observations: (a) HCl is a stronger acid than H2S; (b) H3PO4 is a stronger acid than H3AsO4; (c) HBrO3 is a stronger acid than HBrO2; (d) H2C2O4 is a stronger acid than HC2O4-;...
-
Explain the following observations: (a) HNO3 is a stronger acid than HNO2; (b) H2S is a stronger acid than H2O; (c) H2SO4 is a stronger acid than HSO4-; (d) H2SO4 is a stronger acid than H2SeO4; (e)...
-
Explain which compound is the stronger acid: (a) CHF2CO2HorCH2FCO2H (b) CHF2CO2HorCHBr2CO2H (c) CH3OCH2CO2HorCH3CO2H
-
______________ is an approach to doing business that attempts to maximize an organization's competitiveness through the continual improvement of the quality of its products, services, people,...
-
Business Wear Fashions manufactures one type of women's jacket, production in jobs to fill each customer order. These jackets are supplied to various department stores, and Business Wear sews the...
-
On January 2, 2016, Pop Corporation enters into a business combination with Son Corporation in which Son is dissolved. Pop pays $1,650,000 for Son, the consideration consisting of 66,000 shares of...
-
E 5-8 Downstream sale of inventory Wikan Tbk acquired 80 percent ownership of Budi Tbk several years ago at book value. During 2014, Wikan Tbk sold merchandise to Budi Tbk for $1,000,000 at a gross...
-
Will the future value be larger or smaller if we compound an initial amount more often than annually, for example, every 6 months, or semiannually, holding the stated interest rate constant? Why?...
-
A firm had a net income of $100 per share last year, with a retention rate of 10%. In the first day of the current year, this firm is considering increasing its retention rate to 20%. This would...
-
What advice would you give to a business colleague who is about to start a new high-tech firm that has developed a new accessory for computer tablets? Would you recommend that s/he seek a patent...
-
Show the resonance structures for the conjugate base of the Meta isomer of nitro-phenol and confirm that the nitro group is less effective at stabilizing this anion than it is in the case of the Para...
-
Explain which compound is the weaker base. NH or NH NO b) or
-
What is the APT? In what ways is it both better or worse than the IAPM?
-
Your friend Amber has approached you seeking advice concerning two investment opportunities that she is presently considering. Her classmate Simone has asked her for a loan of $5,000 to help...
-
Please read the following carefully. For each question on the exam, you should assume that: 1. unless expressly stated to the contrary, all events occurred in ?the current taxable year;? 2. all...
-
The pulse rates of 152 randomly selected adult males vary from a low of 37 bpm to a high of 117 bpm. Find the minimum sample size required to estimate the mean pulse rate of adult males. Assume that...
-
Can I get clear explanation how to work these. Thanking you in advance. 1. A rod 12.0 cm long is uniformly charged and has a total charge of -23.0 uC. Determine the magnitude and direction of the...
-
Poll Results in the Media USA Today provided results from a survey of 1144 Americans who were asked if they approve of Brett Kavanaugh as the choice for Supreme Court justice. 51% of the respondents...
-
3.3 Why is it necessary for a firm to have a number of separate ledger accounts in its books of account?
-
Difference between truncate & delete
-
Evaluate IM ( +]. (i + j) i=1 F-1 Fl
-
Draw the following molecule as a line-bond structure, and show how it can be prepared from a ketone and an amine.
-
Show how you could prepare the following compounds from 4-methyl-3-penten-2-one, (CH3)2C =CHCOCH3. (b) (c) CHCCHCH2C (a) CH CH CHCH2H2H CHH2H
-
Show all the steps in the acid-catalyzed formation of a cyclic acetal from ethylene glycol and an aldehyde or ketone.
-
You borrowed $15,000 for buying a new car from a bank at an interest rate of 12% compounded monthly. This loan will be repaid in 48 equal monthly installments over four years. Immediately after the...
-
Discuss how debt restructuring, settlement, or modification works. Discuss the journal entries for debtor and creditor
-
Could CNL be a viable business? If so, under what conditions and what level of production (and, since production is directly related to production workers, employees)? All information provided for...

Study smarter with the SolutionInn App


