Question: Explain which of these compounds is the weaker base? :Z 0 HIN:
Explain which of these compounds is the weaker base?
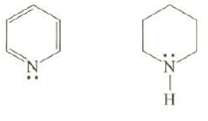
:Z 0 HIN:
Step by Step Solution
3.37 Rating (169 Votes )
There are 3 Steps involved in it
The basic electrons are on a nitrogen atom in both ... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
15-C-O-A-B-R (39).docx
120 KBs Word File


