Explain why the reaction of the cis-isomer of this compound with potassium tert-but oxide in tert-butanol is
Question:
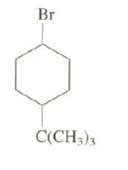
Explain why the reaction of the cis-isomer of this compound with potassium tert-but oxide in tert-butanol is about 500 times faster than that of thetrans-isomer.
Transcribed Image Text:
Br C(CH3)3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (14 reviews)
The most stable chair conformation of the trans isomer has b...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Explain why the reaction shown in eq. 7.19 occurs much more easily than the reaction (That is, why is it necessary to protonate the alcohol before ionization can occur?) (CH) C-OH(CHCHO
-
Explain why compound A reacts faster than compound B when they undergo solvolysis in aqueous acetone. CH C-Cl CH3 CH CH
-
a. Explain why the reaction of an alkyl halide with ammonia gives a low yield of primary amine. b. Explain why a much better yield of primary amine is obtained from the reaction of an alkyl halide...
-
A major electronics manufacturer expects to generate additional revenue from its recently won government contract. The company forecasts that the revenue will be $190 million in the first year, but...
-
Refer to the 'Real life scenario describing the cost of Australian wine. Do you think Australian winemakers use job costing, process costing or a combination of these two product costing systems?...
-
The following financial statement information is from five separate companies. Required 1. Answer the following questions about Company A. a. What is the amount of equity on December 31, 2016? b....
-
What do many experts agree is the greatest threat to the success of any project? a. lack of proper funding b. a failure to communicate c. poor listening skills d. inadequate staffing LO.1
-
Nathan Detroit owns 400 shares of the food company General Mills, Inc., which he purchased during the recession in January 2009 for $35 per share. General Mills is regarded as a relatively safe...
-
Basu Corporation had the following net income (loss) for the first three years of operations, respectively: $6,900, ($1,800), and $2,600. If the Retained Earnings balance at the end of year three is...
-
Use Apples financial statements in Appendix A to answer the following. 1. Compute Apples return on total assets for the years ended September 28, 2019, and September 29, 2018. 2. Is the change in...
-
Show the product of thisreaction: Ph Br- - + NaOEt Br EIOH Ph
-
Explain which of these compounds has a faster rate of E2elimination: CH CH3 CI 'CI
-
Represent the following situations diagrammatically: (a) An economy in which AD increases as the economy is self-regulating out of a recessionary gap, (b) An economy in which AD decreases as the...
-
The following data apply to Superior Auto Supply Inc. for May 2011. 1. Balance per the bank on May \(31, \$ 8,000\). 2. Deposits in transit not recorded by the bank, \(\$ 975\). 3. Bank error; check...
-
How do you determine whether there is a linear correlation between two variables \(x\) and \(y\) ? Use Table 14.10. Table 14. 10 n a = 0.05 0.950 0.878 4 5 6 0.811 7 0.754 8 0.707 9 0.666 10 0.632 11...
-
Comparative Analysis Problem: Columbia Sportswear Company vs. Under Armour, Inc. The financial statements for the Columbia Sportswear Company can be found in Appendix A and Under Armour, Inc.'s...
-
The following information is available for Book Barn Company's sales on account and accounts receivable: After several collection attempts, Book Barn wrote off \(\$ 4,500\) of accounts that could not...
-
The following information comes from the accounts of Jersey Company: Required a. There were \(\$ 170,000\) of sales on account during the accounting period. Write-offs of uncollectible accounts were...
-
Which accounting standards are statutory/mandatory requirements of the corporate sector?
-
Which of the ocean zones shown would be home to each of the following organisms: lobster, coral, mussel, porpoise, and dragonfish? For those organisms you identify as living in the pelagic...
-
Find the slope of each line. 4 x
-
Is a nucleus that absorbs at 6.50 more shielded or less shielded than a nucleus that absorbs at 3.20 ? Does the nucleus that absorbs at 6.50 require a stronger applied field or a weaker applied...
-
Identify the indicated sets of protons as unrelated, homotopic, enantiotopic, ordiastereotopic: (a) (b) C (c)
-
How many types of nonequivalent protons are present in each of the followingmolecules? (c) (b) CH3CH2CH20CH3 (a) H3C CH3 Naphthalene (e) (d) C=CH2 CO2CH2CH3 Ethyl acrylate Styrene
-
A company is evaluating a new 4-year project. The equipment necessary for the project will cost $3,300,000 and can be sold for $650,000 at the end of the project. The asset is in the 5-year MACRS...
-
You have just been hired as a new management trainee by Earrings Unlimited, a distributor of earrings to various retail outlets located in shopping malls across the country. In the past, the company...
-
I need to see where the calculations for this problem come from plz. 5. Award: 4.00 points Lucido Products markets two computer games: Claimjumper and Makeover. A contribution format income statement...

Study smarter with the SolutionInn App


