Figure gives the energy levels for an electron trapped in a finite potential energy well 450eV deep.
Question:
Figure gives the energy levels for an electron trapped in a finite potential energy well 450eV deep. If the electron is in the n = 3 state, what is its kineticenergy?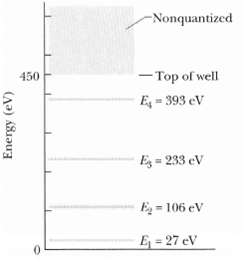
Transcribed Image Text:
-Nonquantized - Top of well 450 E 393 eV E = 233 eV Eg = 106 eV E=27 eV Energy (eV)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 55% (9 reviews)
From figure we see that the sum of the kinetic and potential energies in that p...View the full answer

Answered By

Charles mwangi
I am a postgraduate in chemistry (Industrial chemistry with management),with writing experience for more than 3 years.I have specialized in content development,questions,term papers and assignments.Majoring in chemistry,information science,management,human resource management,accounting,business law,marketing,psychology,excl expert ,education and engineering.I have tutored in other different platforms where my DNA includes three key aspects i.e,quality papers,timely and free from any academic malpractices.I frequently engage clients in each and every step to ensure quality service delivery.This is to ensure sustainability of the tutoring aspects as well as the credibility of the platform.
4.30+
2+ Reviews
10+ Question Solved
Related Book For 

Probability & Statistics For Engineers & Scientists
ISBN: 9780130415295
7th Edition
Authors: Ronald E. Walpole, Raymond H. Myers, Sharon L. Myers, Keying
Question Posted:
Students also viewed these Modern Physics questions
-
The penetration distance ? in a finite potential well is the distance at which the wave function has decreased to 1/e of the wave function at the classical turning point: The penetration distance can...
-
Discuss what happens to the energy levels for an electron trapped in a one-dimensional box as the length of the box increases.
-
Suppose that an electron trapped in a one-dimensional infinite well of width 250 pm is excited from its first excited state to its third excited state. (a) What energy must be transferred to the...
-
Toby dies on 2 March 2021, leaving an estate valued at 400,000. None of the transfers made on death are exempt from IHT. Calculate the IHT due on the estate if the total of the gross chargeable...
-
Talk to a financial planner or an insurance agent about the financial difficulties faced by people who lack adequate home and auto insurance. What common coverages do many people overlook?
-
The dynamic model between an output variable y and an input variable u can be expressed by (a) Does this system exhibit an oscillatory response after an arbitrary change in u? (b) What is the...
-
Develop a simulation model for a 3-year financial analysis of total profit based on the following data and information. Sales volume in the first year is estimated to be 100,000 units and is...
-
Commonwealth v. Shea (see Appendix A)brief only the issue of whether the ocean can be considered a deadly weapon
-
Hodgkiss Corporation is evaluating an extra dividend versus a share repurchase. In either case, $23,940 would be spent. Current earnings are $3.00 per share, and the stock currently sells for $93 per...
-
Modify the Student class in Listing 8.2 so that it implements the comparable interface. Define the compareTo method to order Student objects based on the value in studentNumber. In a main method...
-
An electron in the n = 2 state in the finite potential well of figure absorbs 400eV of energy from an external source. Using the energy-level diagram of figure, determine the electron's kinetic...
-
(a) Show that for the region x > L in the finite potential well of figure ?(x) = De2ks is a solution of Schrodinger's equation in its one-dimensional form, where D is a constant and k is positive.(b)...
-
One problem with the design is that about 20% of the women who are randomized to PMH will not comply (i.e., will go off PMH during the trial). In addition, 10% of the participants in the control...
-
PART 1: DIGITAL TECHNOLOGY: Describe the key digital technology groups studied in this course and include a discussion of two examples for each group. PART 2: SOCIAL MEDIA: As studied in this course,...
-
Doing a strategic analysis of GraceKennedy Limited, What is the current level of its economic performance, an indication of the factors responsible for the current performance and recommendations for...
-
Dynamic capability is the ability for change and manage corporate learning. It allows an enterprise to adapt, develop and respond to future opportunities and discontinuous technologies. Innovation...
-
What potential solutions can organizations try to help support the adoption of a CDSS? In other words, what are some ways an organization can address the factors of implementation obstruction that...
-
Identify and briefly describe and discuss the three most important factors in building and maintaining trust among virtual global team members. Include in your discussion how you can leverage these...
-
Measure and evaluate the risk of revenue loss
-
Havel says the grocer doesnt believe what is on the sign and indeed, he says the grocers customers will barely notice it. But Havel maintains that the sign serves a specific function. How would you...
-
In which of the following molecules would you expect to find delocalized molecular orbitals? Explain. (a) C 2 H 4 ; (b) SO 2 ; (c) H 2 CO.
-
The following is the distribution of the readings obtained with a Geiger counter of the number of particles emitted by a radioactive substance in 100 successive 40-second intervals: (a) Verify that...
-
The following are the hours of operation to failure of 38 light bulbs. Use a suitable statistical computer program to test whether the mean failure time of such light bulbs is significantly less than...
-
The following are the hours of operation to failure of 38 light bulbs. Use a suitable statistical computer program to test whether the mean failure time of such light bulbs is significantly less than...
-
You have $55,000. You put 15% of your money in a stock with an expected return of 10%, $38,000 in a stock with an expected return of 18%, and the rest in a stock with an expected return of 22%. What...
-
Portfolio return and beta Personal Finance Problem Jamie Peters invested $ 1 1 3 , 0 0 0 to set up the following portfolio one year ago: a . Calculate the portfolio beta on the basis of the original...
-
. Emerson Cammack wishes to purchase an annuity contract that will pay him $7,000 a year for the rest of his life. The Philo Life Insurance Company figures that his life expectancy is 20 years, based...

Study smarter with the SolutionInn App


