Question: Figure shows the first four peaks of the x-ray diffraction pattern for copper, which has an FCC crystal structure; monochromatic x-radiation having a wavelength of
Figure shows the first four peaks of the x-ray diffraction pattern for copper, which has an FCC crystal structure; monochromatic x-radiation having a wavelength of 0.1542 nm was used.
(a) Index (i.e., give h, k, and l indices) for each of these peaks.
(b) Determine the interplanar spacing for each of the peaks.
(c) For each peak, determine the atomic radius for Cu and compare these with the value presented in Table3.1.
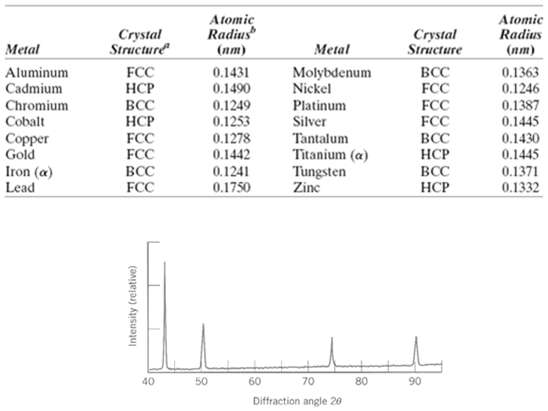
Atomic Radius Atomic Radius Crystal Structure Crystal Structure Metal Metal () () 0.1431 0.1490 BCC FCC Aluminum Cadmium FCC Molybdenum Nickel 0.1363 0.1246 BCC FCC FCC Platinum Silver Chromium Cobalt 0.1249 0.1253 0.1278 0.1442 FCC FCC 0.1387 0.1445 Tantalum Titanium (a) Tungsten Zine 0.1430 0.1445 0.1371 Copper Gold BCC Iron (a) BCC 0.1241 BCC 0.1332 Lead FCC 0.1750 40 50 60 70 80 90 Diffraction angle 20 Intensity (relative)
Step by Step Solution
3.33 Rating (174 Votes )
There are 3 Steps involved in it
a Since Cu has an FCC crystal structure only those peaks ... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
33-E-M-S-E-M-S (89).docx
120 KBs Word File


