Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Q15 * Your answer is incorrect. Figure below shows the first four peaks of the x-ray diffraction pattern for copper, which has an FCC crystal
Q15 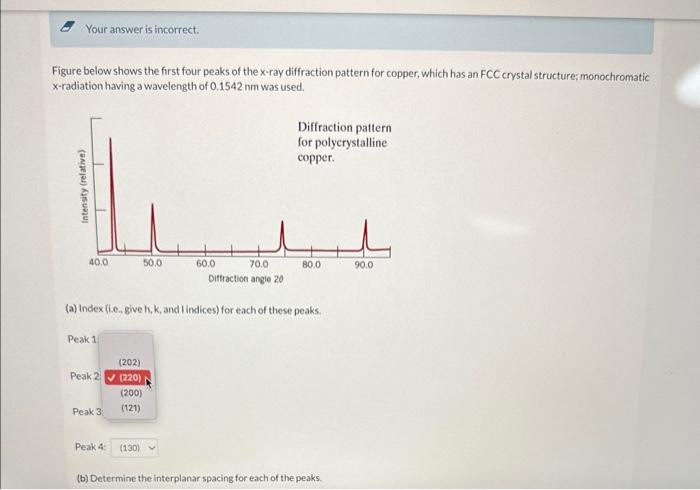

* Your answer is incorrect. Figure below shows the first four peaks of the x-ray diffraction pattern for copper, which has an FCC crystal structure; monochromatic x-radiation having a wavelength of 0.1542nm was used. (a) index (i.e. give h, k, and lindices) for each of these peaks. Peak 1 Peak 4: (b) Determine the interplanar spacing for each of the peaks. (b) Determine the interplanar spacing for each of the peaks. Peak 1: Peak 2 Peak 3: nm Peak 4: nm (c) For each peak, determine the atomic radius for Cu and compare these with the value presented in Table Atomic Radii and Crystal Structures for 16 Metals 

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


