Find the value of n?in Equation 38-32 that gives the measured dissociation energy of 741 kJ/mol for
Question:
Find the value of n?in Equation 38-32 that gives the measured dissociation energy of 741 kJ/mol for LiCl, which has the same structure as NaCl and for which r0 = 0.257 nm.
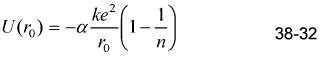
Transcribed Image Text:
ke? (1-) U(r.) = -a- 38-32
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 72% (11 reviews)
Using Equations 3832 and 38...View the full answer

Answered By

HARSH RANJAN
Taken classes at college to graduates, Also worked as an expert to a freelancer online question-solving portal for more than 8 months with an average rating greater than 4.2 out of 5.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Fundamentals of Ethics for Scientists and Engineers
ISBN: 978-0195134889
1st Edition
Authors: Edmund G. Seebauer, Robert L. Barry
Question Posted:
Students also viewed these Modern Physics questions
-
Find the value of n in each proportion. a. 2.54cm/1in = n/12in b. 1km/0.625mi = n/200mi c. 1yd/0.96m = 140yd/n
-
Find the value of permutation. 6P2
-
Find the value of permutation. 7P2
-
In a 1994 study, 164 pregnant, HIV-positive women were randomly assigned to receive the drug AZT during pregnancy and 160 such women were randomly assigned to a control group that received a placebo....
-
Most companies use the voucher system. Due to poor profitability, several employees in the accounting office were let go. Joe Rose, who handled the verification of the vouchers in the voucher...
-
Read the below context, discuss what leadership style can be used to resolve this situation and how it will affect your change management process. Your organisation is shifting their document storage...
-
Understanding cultural diversity is crucial. Work by Hofstede has identified four distinguishing factors of national culture: individualism, power distance, uncertainty avoidance and masculinity. He...
-
Prestopino Corporation produces motorcycle batteries. Prestopino turns out 1,500 batteries a day at a cost of $6 per battery for materials and labor. It takes the firm 22 days to convert raw...
-
Explain the importance of decision making for managers at each of the three primary organization levels along with the associated decision characteristics
-
Red Bridges Towing is owned by Ken Cordial. The company has a June 30 fiscal year end and prepares adjustments on an annual basis. The following is an alphabetical list of its accounts at June 30,...
-
The distance between the Li+ and Cl ions in LiCl is 0.257 nm. Use this and the molecular mass of LiCl (42.4 g/mol) to compute the density of LiCl.
-
Suppose identical bowling balls of radius R are packed into a hexagonal close-packed structure. What fraction of the available volume of the unit cell is filled by the bowling balls?
-
Which of the following is not a characteristic of the lower control risk approach? a. Control risk is usually assessed at maximum level. b. Substantive tests are usually restricted. c. The auditor...
-
A storeroom is used to organize items stored in it on N shelves. Shelves are numbered from 0 to N-1. The K-th shelf is dedicated to items of only one type, denoted by a positive integer A[K]....
-
CASES CASE 10.1 Money in Motion Jake Nguyen runs a nervous hand through his once finely combed hair. He loosens his once perfectly knotted silk tie. And he rubs his sweaty hands across his once...
-
(3.8) Axiom, Definition of false false = true (3.9) Axiom, Distributivity of over : (pq) p=q
-
The board of directors of Unilever has been impressed by the presentation you did, and they further instructed you to conduct a more insightful investigation about the Sri Lankan market. They have...
-
The sample space listing the eight simple events that are possible when a couple has three children is {bbb, bbg, bgb, ogg, gbb, gbg, ggb, ggg}. After identifying the sample space for a couple having...
-
The city of South Bend, Indiana, adopted an affirmative action plan to give preference to minorities in hiring and promotion for police and firefighter positions. The affirmative action plan was...
-
Discuss the information available from the following techniques in the analysis of inorganic pigments used in antique oil paintings: (i) Powder X-ray diffraction, (ii) Infrared and Raman...
-
Using a single op amp, design an amplifier with a gain of v 2 /v 1 = 3/4, input resistance of 8 k, and zero output resistance.
-
In situations where a small signal must travel over a distance, a shielded cable is used in which the signal wire is surrounded by an insulator and then enclosed by a cylindrical conductor carrying...
-
What is the advantage of placing the two insulated electric wires carrying as close together of even twisted about each other?
-
Explain why, exactly the lights may dim briefly when a refrigerator motor starts up. When an electric heater is turned on, the lights may stay dimmed as long as the heater is on. Explain the...
-
A government bond matures in 30 years, makes semi-annual coupon payments of 6.0% ($120 per year) and offers a yield of 3.7% annually compounded. Assume face value is $1,000. Three years later the...
-
Your objective is: 1. Carry out a life insurance needs analysis, for each one of them (show your calculations) [30 Marks] 2. Refer to the case and the insurance plan quotes. Would you recommend...
-
TufStuff, Incorporated, sells a wide range of drums, bins, boxes, and other containers that are used in the chemical industry. One of the company s products is a heavy - duty corrosion - resistant...

Study smarter with the SolutionInn App


