For the ZrO 2 ?CaO system (Figure), write all eutectic and eutectoid reactions for cooling. Composition (mol%
Question:
For the ZrO2?CaO system (Figure), write all eutectic and eutectoid reactions for cooling.
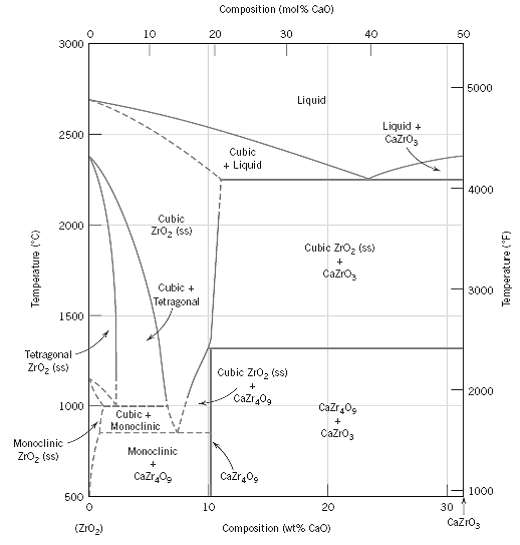
Transcribed Image Text:
Composition (mol% Cao) 10 20 30 40 50 3000 5000 Liquid Liquid + Cazro, 2500 Cubic + Liquid 4000 Cubic 2000 Zro, (ss) Cubic Zro, (ss) Cazro, Cubic + 3000 Tetragonal 1500 Tetragonal Zroz (ss) Cubic ZIO, (ss) 2000 Cazr 0g 1000 Cazr,0g Cubic + Monoclinic Cazro3 Monoclinic Monoclinic ZrOz (ss) Cazr Og Cazr0g 1000 500 10 20 30 CazrO3 Composition (wt% CaO) (Z1O2) (0.) eameredwejL Temperature (F)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 57% (14 reviews)
There is only one eutectic for the portion of the ZrO 2 CaO system shown in ...View the full answer

Answered By

Shristi Singh
A freshman year metallurgy and material science student in India.
4.80+
2+ Reviews
10+ Question Solved
Related Book For 

Fundamentals of Materials Science and Engineering An Integrated Approach
ISBN: 978-1118061602
4th Edition
Authors: David G. Rethwisch
Question Posted:
Students also viewed these Materials Science Engineering questions
-
For the ZrO2-CaO system (Figure 12.26), write all eutectic and eutectoid reactions for cooling.
-
Write the eutectic reaction that occurs, including the compositions of the three phases in equilibrium, and calculate the amount of and in the eutectic micro constituent in the Mg-Al system (Figure...
-
Figure 11-32 shows a cooling curve for an Al-Si alloy and Figure 11-19 shows the binary phase diagram for this system. Determine (a) The pouring temperature; (b) The superheat; (c) The liquidus...
-
Reading material Raymond Frost, Alexa K. Fox & Judy Strauss (2019). Product: The Online Offer. E-Marketing , 9, 206 - 228. Raymond Frost, Alexa K. Fox & Judy Strauss (2019). Price: The Online Value....
-
Discuss in depth the demographer of Germany. Outline the discuss by take in to account the following factors Population, financial status, top 3 diseases and overall healthcare, type of healthcare...
-
The classified balance sheet and selected income statement data for Amarillo Auto Supply, Inc., as of December 31, 2018, are presented next. Selected Income Statement Data Gross...
-
Common Size Statements (LO3, 4) . Comparative balance sheets for Albany, Inc., follow for year-end 2012 and 2011. Its president is concerned about the decline in total assets and wants to know where...
-
As a manager employed by Want-It-Now Rapid Delivery Service, you are responsible for pricing the services involving same-day deliveries. Among your primary concerns is the competitive aspects of your...
-
You bought one share of stock of Peace-n-Joy Jam Inc. at a price of $98. You also sold a call option on this stock with a strike of $100. You received a premium of $3.50. What should the stock price...
-
W. C. Sanders, owner of Fort Engines, a producer of heavy-duty snow blower engines, needs to develop an aggregate plan for the coming year. The company currently uses 20 individuals working 160...
-
What point defects are possible for Al2O3 as an impurity in MgO? How many Al3+ ions must be added to form each of these defects?
-
From Figure, the phase diagram for the MgO???Al2O3 system, it may be noted that the spinel solid solution exists over a range of compositions, which means that it is nonstoichiometric at compositions...
-
Explain why each of the following is recognised as a provision in the statement of financial position (balance sheet) of a telecommunications company: (a) On 15 December Year 2, the Group announced a...
-
Assume that John wants to annuitize the annuity and is told that he can receive a straight life annuity for $600 a month for life. If the actuarial number of payments is 300, how much of the first...
-
An epidemiologist plans to conduct a survey to estimate the percentage of women who give birth. How many women must be surveyed in order to be 90% confident that the estimated percentage is in error...
-
Your homework for this week is to watch the first lecture on Financial Accounting and at the end of the outline there are several problems for you to do. The problems begin with parts A-D for you to...
-
Sheril Rose was a brilliant but penniless material scientist. She had designed a new type of solar panel she believed had great commercial potential. On January 15, she approached Felda Higgins, a...
-
IAS 23 requires companies to capitalize borrowing costs directly attributable to the acquisition, construction or production of an asset into the cost of an asset.Previously, accounting standard...
-
What is an opportunity cost? How does it differ from an ordinary accounting cost?
-
A report from the college dean indicates that for the previous semester, the grade distribution for the Department of Psychology included 135 As, 158 Bs, 140 Cs, 94 Ds, and 53 Fs. Determine what kind...
-
H vap = 31.3 kJ/mol for acetone. If 1.40 kg of water were vaporized to steam in a boiler, how much acetone (in kg) would need to be vaporized to use the same amount of heat?
-
How does a metal increase its internal energy during plastic deformation?
-
In what ways can recrystallization be used to enable large amounts of deformation without fear of fracture?
-
What is the major distinguishing feature between hot and cold working?
-
On February 1, 2021, Arrow Construction Company entered into a three-year construction contract to build a bridge for a price of $8,600,000. During 2021, costs of $2,200,000 were incurred with...
-
Salespersons' Report and Analysis Walthman Industries Inc. employs seven salespersons to sell and distribute its product throughout the state. Data taken from reports received from the salespersons...
-
Stockholders do not have the power to bind the corporation to contracts. This is referred to as lack of mutual agency. True false question. True False

Study smarter with the SolutionInn App


