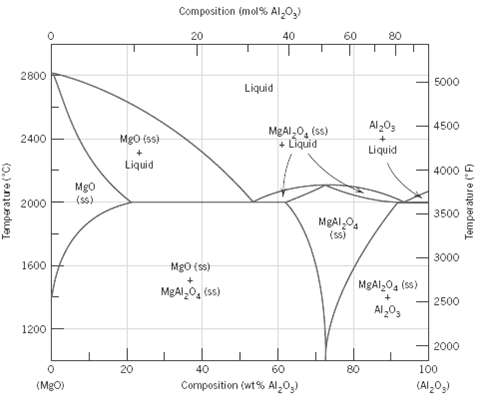
Question: From Figure, the phase diagram for the MgO???Al2O3 system, it may be noted that the spinel solid solution exists over a range of compositions, which
From Figure, the phase diagram for the MgO???Al2O3 system, it may be noted that the spinel solid solution exists over a range of compositions, which means that it is nonstoichiometric at compositions other than 50 mol% MgO???50 mol% Al2O3.
(a) The maximum nonstoichiometry on the Al2O3-rich side of the spinel phase field exists at about 2000°C (3630°F) corresponding to approximately 82 mol% (92 wt%) Al2O3. Determine the type of vacancy defect that is produced and the percentage of vacancies that exist at this composition.
(b) The maximum nonstoichiometry on the MgO-rich side of the spinel phase field exists at about 2000°C (3630°F) corresponding to approximately 39 mol% (62 wt%) Al2O3. Determine the type of vacancy defect that is produced and the percentage of vacancies that exist at thiscomposition.
Composition (mol% Al,0,) 40 60 80 2800 5000 Liquid Al 203 4500 MgAl 0, (ss) + Liquid 2400 Mg0 (ss) Liquid Liquid 4000 Mg0 (so) 2000 3500 MgAI,O, (ss) 3000 1600 Mgo (ss) MgAl,0, (ss) MgAl,0, (ss) 2500 Al0, 1200 2000 20 40 60 80 100 (Al0,) Composition (wt % Al,0,) (MgO) Temperature ('C) Temperature ("F)
Step by Step Solution
3.51 Rating (168 Votes )
There are 3 Steps involved in it
a For this portion of the problem we are to determine the type of vacancy defect that is produced on the Al 2 O 3 rich side of the spinel phase field ... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
33-E-M-S-E-M-S (493).docx
120 KBs Word File


