From what alkyne might each of the following substances have been made? (Yellow-green =Cl) (b) (a)
Question:
From what alkyne might each of the following substances have been made? (Yellow-green =Cl)
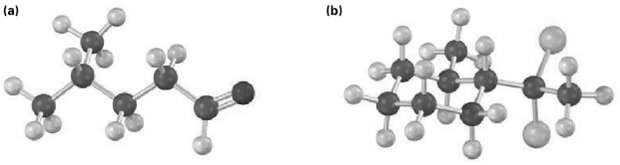
Transcribed Image Text:
(b) (a)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 45% (11 reviews)
CH3 CH3CHCHCCH 4Methyl1pentyne An al...View the full answer

Answered By

Ashington Waweru
I am a lecturer, research writer and also a qualified financial analyst and accountant. I am qualified and articulate in many disciplines including English, Accounting, Finance, Quantitative spreadsheet analysis, Economics, and Statistics. I am an expert with sixteen years of experience in online industry-related work. I have a master's in business administration and a bachelor’s degree in education, accounting, and economics options.
I am a writer and proofreading expert with sixteen years of experience in online writing, proofreading, and text editing. I have vast knowledge and experience in writing techniques and styles such as APA, ASA, MLA, Chicago, Turabian, IEEE, and many others.
I am also an online blogger and research writer with sixteen years of writing and proofreading articles and reports. I have written many scripts and articles for blogs, and I also specialize in search engine
I have sixteen years of experience in Excel data entry, Excel data analysis, R-studio quantitative analysis, SPSS quantitative analysis, research writing, and proofreading articles and reports. I will deliver the highest quality online and offline Excel, R, SPSS, and other spreadsheet solutions within your operational deadlines. I have also compiled many original Excel quantitative and text spreadsheets which solve client’s problems in my research writing career.
I have extensive enterprise resource planning accounting, financial modeling, financial reporting, and company analysis: customer relationship management, enterprise resource planning, financial accounting projects, and corporate finance.
I am articulate in psychology, engineering, nursing, counseling, project management, accounting, finance, quantitative spreadsheet analysis, statistical and economic analysis, among many other industry fields and academic disciplines. I work to solve problems and provide accurate and credible solutions and research reports in all industries in the global economy.
I have taught and conducted masters and Ph.D. thesis research for specialists in Quantitative finance, Financial Accounting, Actuarial science, Macroeconomics, Microeconomics, Risk Management, Managerial Economics, Engineering Economics, Financial economics, Taxation and many other disciplines including water engineering, psychology, e-commerce, mechanical engineering, leadership and many others.
I have developed many courses on online websites like Teachable and Thinkific. I also developed an accounting reporting automation software project for Utafiti sacco located at ILRI Uthiru Kenya when I was working there in year 2001.
I am a mature, self-motivated worker who delivers high-quality, on-time reports which solve client’s problems accurately.
I have written many academic and professional industry research papers and tutored many clients from college to university undergraduate, master's and Ph.D. students, and corporate professionals. I anticipate your hiring me.
I know I will deliver the highest quality work you will find anywhere to award me your project work. Please note that I am looking for a long-term work relationship with you. I look forward to you delivering the best service to you.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
What type of solid will each of the following substances form? a. CO2 b. SiO2 c. Si d. CH4 e. Ru f. I2 g. KBr h. H2O i. NaOH j. U k. CaCO3 l. PH3 m. GaAs n. BaO o. NO p. GeO2
-
Each of the following substances is dissolved in a separate 10.0-L container of water: 1.5 mol NaCl, 1.3 mol Na2SO4, 2.0 mol MgCl2, and 2.0 mol KBr. Without doing extensive calculations, rank the...
-
What role might each of the following play in contributing to income inequality: (a) Risk taking, (b) Education, (c) Innate abilities and attributes?
-
Blocks A and B shown in the figure are free and all surfaces are non-friction. Find the velocity of the blocks at t = 3 s, since the tensile force shown in the graphic is applied to the block A...
-
You have been asked to evaluate the following investment opportunity. A small firm is available for purchase at an initial cost of $150,000, to be paid to the current owner in equal installments over...
-
Two circular loops lie side by side in the same plane. One is connected to a source that supplies an increasing current; the other is a simple closed ring. Is the induced current in the ring in the...
-
Prepare an issue log for the project. Include past issues discussed in prior chapters, such as Yusaff leaving the company, a difficult and vocal member of the user group, and unproductive meetings....
-
Green Day Corporation had income from continuing operations of $12,600,000 in 2004. During 2004, it disposed of its restaurant division at an after-tax loss of $189,000. Prior to disposal, the...
-
Over the last year net PP&E for Hershey's has increased from 774 million dollars to 894 million dollars. You also notice that Hershey's reported a depreciation expense of 61 million dollars. What was...
-
Delft House, Inc., a multinational company based in Amsterdam, organizes and coordinates art shows and auctions throughout the world. Its budgeted and actual costs for last year are as follows: Delft...
-
Name the following alkynes, and predict the products of their reaction with (i) H2 in the presence of a Lindlar catalyst and (ii) H3O+ in the presence ofHgSO4: (b) (a)
-
How would you prepare the following substances, starting from any corn- pounds having four carbons orfewer? (a) (b)
-
Onslow Co. purchased a used machine for $178,000 cash on January 2. On January 3, Onslow paid $2,840 to wire electricity to the machine. Onslow paid an additional $1,160 on January 4 to secure the...
-
A retail product has the following standard costs established: Direct Material per unit - 2 pounds at $5 a pound Direct Labor per unit - 3 hours at $12 an hour Manufacturing Overhead - $5 per labor...
-
In a recent year, the Better Business Bureau settled 75% of complaints they received. (Source: USA Today, March 2, 2009) You have been hired by the Bureau to investigate complaints this year...
-
A 1200-ft equal tangent crest vertical curve is currently designed for 50 mph. A civil engineering student contends that 60 mph is safe in a van because of the higher driver's eye height. If all...
-
Required information [The following information applies to the questions displayed below.] Victory Company uses weighted-average process costing to account for its production costs. Conversion cost...
-
Finer, % 100 90 80 70 60 50 40 30 20 10 0 0.01 0.1 1 Size, mm L 10 100 Figure shows a grain size distribution curve of soil. Estimate the coefficient of curvature (Cc) of this soil.
-
What measures, if any, should be taken in handling the seasoned sales manager? LO.1
-
San Carlos Bank and Trust Company uses a credit-scoring system to evaluate most consumer loans that amount to more than $2,500. The key factors used in its scoring system are found at the conclusion...
-
Write a molecular equation for the precipitation reaction that occurs (if any) when each pair of aqueous solutions is mixed. If no reaction occurs, write NO REACTION. a. Sodium chloride and lead(II)...
-
Suppose the migrating methyl group in part (a) were labeled with the hydrogen isotopes deuterium (D) and tritium (T) so that it is a -CHDT group with the S configuration. What would be the...
-
Suppose the migrating methyl group in part (a) were labeled with the hydrogen isotopes deuterium (D) and tritium (T) so that it is a -CHDT group with the S configuration. What would be the...
-
When compound A is irradiated with ultraviolet light for 115 hours in pentane, an isomeric compound B is obtained that decolorizes bromine in CH2C12 and reacts with ozone to give, after the usual...
-
The following are the information of Chun Equipment Company for Year 2 . ( Hint: Some of the items will not appear on either statement, and ending retained earnings must be calculated. ) Salaries...
-
Alta Ski Company's inventory records contained the following information regarding its latest ski model. The company uses a periodic inventory system. Beginning inventory, January 1, 2018 1,250 units...
-
Fibertech GmbH is a distributor of outdoors technical clothing. The company outsources the production of clothing to external manufacturers in Bangladesh and sells the clothing under its own brands....

Study smarter with the SolutionInn App


