From which alkene could each of the following cyclopropane derivatives be prepared using the Simmons- Smith reaction?
Question:
From which alkene could each of the following cyclopropane derivatives be prepared using the Simmons- Smith reaction?
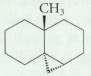
Transcribed Image Text:
CH3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 85% (14 reviews)
Cyclopropane formatio...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
What alkenes would you choose as starting materials in order to prepare each of the following cyclopropane derivatives by reaction with iodomethylzinc iodide?
-
What alkenes would you choose as starting materials in order to prepare the following cyclopropane derivatives by reaction with iodomethylzinc iodide?
-
Specify the appropriate alkene and reagents for synthesis of each of the following alcohols by hydroboration-oxidation. (a) (b) (c) (d) (e) (f) CH 2 OH
-
A coil of wire 0.1 m long and having 15 turns carries a current of 1.0 A. (a) Compute the flux density if the coil is within a vacuum. (b) A bar of an iron-silicon alloy, the B-H behavior for which...
-
New York married couple Harry and Winona receives a promotional flyer in the mail from Desert Land Sales Corp. of Arizona. The flyer advertises undeveloped one-acre sites as part of a 250-acre...
-
Eric Fenson is employed as a shipping supervisor. In theevenings and on weekends, he holds a second job as a real estatesalesman for a national real estate firm. His financial informationfor 2022 i 2...
-
Who are the members of the board of directors? The top management team?
-
Westbrook Furniture uses departmental overhead rates (rather than a plantwide overhead rate) to allocate its manufacturing overhead to jobs. The companys two production departments have the...
-
Compute the spending variance where: Planned activity of 620 units Actual units was 610 Actual cost was 57,000 Cost formula is $1.100 per month plus $10 per unit. 5300U 5200U $200 F $300 F Next...
-
John Parsons (123-45-6781) and George Smith (123-45-6782) are 70% and 30% owners, respectively, of Premium, Inc. (11-1111111), a candy company located at 1005 16th Street, Cut and Shoot, TX 77303....
-
What substitution and elimination products (if any)might be obtained when each of the following alkyl halides is treated with sodium methoxide in methanol? (a) methyl iodide (b)...
-
Choose the alkyl halide(s) frorn the following list of C6H13Br isomers that meet each criterion below. (1) l-bromohexane (2) 3 -bromo-3 -methylpentane (3 ) I -bromo-2,2-dimethylbutane (4) 3 -bromo-2-...
-
Ready Corporation began the month of June with $280,000 of current assets, a current ratio of 2.8:1, and an acid-test ratio of 1.2:1. During the month, it completed the following transactions (the...
-
You are the manager of internal audit of Coverit Corporation, a large insurance company. One day you receive an urgent letter from the controller expressing his concerns about some organizational...
-
Daintree Ltd. is a large retailer that operates department stores in all major cities throughout Australia. Recently it has expanded its operations into Southeast Asia. Although each store operates...
-
Draw two points P and Q. Then sketch PQ. Add a point R on the ray so that Q is between P and R. C D A B FL E
-
Hypothesis testing and testing claims with confidence intervals are two different approaches that lead to the same conclusion. In the following activities, you will compare and contrast those two...
-
The following system of periodic tasks is scheduled and executed according to a cyclic schedule. Draw an execution trace (timeline) showing two occurances of each task. Ti ei Pi 1 8 T2 4 15 T3 3 20...
-
What is the difference between the expected rate of return and the realized rate of return?
-
The baseball player A hits the ball from a height of 3.36 ft with an initial velocity of 34.8 ft/s. 0.14 seconds after the ball is hit, player B who is standing 15 ft away from home plate begins to...
-
The mass spectrum of tetramethylsilane, (CH 3 ) 4 Si, has a base peak at m/z = 73. Calculate the relative abundances of the isotopic peaks at m/z = 74 and 75.
-
Match the IR spectrum in Fig. 12.13 to one of the following three compounds: 2-methyl-1-octene, butyl methyl ether, or 1-pentanol. FIGURE 12.13 The IR spec- trum for Problem 12.13. percent...
-
In the infrared spectrum of nonane in Fig. 12.4, what is the absorbance of the sharp peak at 1380 cm 1 ? percent transmittance 100 80 60 40 20 0 2.6 2.8 3 wavelength, micrometers 3.5 4 4.5 5 5.5 6 7...
-
Mass LLp developed software that helps farmers to plow their fiels in a mannyue sthat precvents erosion and maimizes the effoctiveness of irrigation. Suny dale paid a licesnsing fee of $23000 for a...
-
Average Rate of Return The following data are accumulated by Lone Peak Inc. in evaluating two competing capital investment proposals: 3D Printer Truck Amount of investment $40,000 $50,000 Useful life...
-
4. (10 points) Valuation using Income Approach An appraiser appraises a food court and lounge and provides the following assessment: o O The building consists of 2 floors with the following (6)...

Study smarter with the SolutionInn App


