Give the structure of the nucleophile that could be used to convert iodoethane into each of the
Question:
Give the structure of the nucleophile that could be used to convert iodoethane into each of the following compounds in an SN2 reaction.
(a) CH3CH2CN
(b)
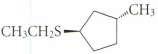
Transcribed Image Text:
CH3 alas-Cal, CH CH2S
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 58% (12 reviews)
a Na...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Give the structure of each product. LI iAlli, o exCeSS H Ni, heat a. CH3C b. CH- CH-CH-O CH-CH- CH3 1. NaBH 2. H2O, H 10% Jones reagent C. d. Ag,O e. CH,CH=CHCHO
-
Give the structure of the that would with mCPBA to give each of the following expoxides. (a) (b) . /A C CH2 H,C C-4 CH,
-
Give the structure$ of the starting materials that would yield each of the compounds below in Diels-Alder reactions. Pay careful attention to stereochemistry, where appropriate. (a) (b) ,, , ,
-
Repeat Example 13.1, but for electrophilic substitution at C-2 or C-4 of pyridine. Explain why substitution at C-3 (eq. 13.2) is preferred. 4 H
-
How would a marketing manager or management team overcome the difficulties faced in trying to ensure ethical standards at the micro or macro level of marketing?
-
Varian Company uses the weighted average method for its process costing system. The Molding Department at Varian began the month of January with 80,000 units in Work in Process Inventory, all of...
-
Does this seem appropriate for the situation the organization is in now?
-
Using the SoakNFun Swim Park information presented, do the following tasks. SoakNFun Swim Park sells individual and family tickets. With a ticket, each person receives a meal, three beverages, and...
-
Ron Corporation had 8 million shares of common stock outstanding during the current calendar year. On July 1, Ron issued ten thousand $1,000 face value, convertible bonds. Each bond is convertible...
-
C&C Properties is an S corporation that owns two rental real estate undertakings: Carrot Plaza and Cantaloupe Place. Both properties produce an annual $10,000 operating loss. C&Cs Schedule K...
-
Rank the following compounds in orcler of increasing SN2 reaction rate with KI in acetone. methyl bromide sec-butyl bromide 3-(bromomethyl)-3-methylpentane I-bromopentane -bromo-2-methylbutane
-
Tell wbether each of the following reactions favors reactants or products at equilibrium. (Assume that all reactants and products are soluble.) (a) CH3CI + I- CH3I + CI- (b) CH3CI + -OCH3 CH3OCH3 +...
-
1. The University of Pittsburgh Medical Center (UPMC) relies on information systems to operate 19 hospitals, a network of other care sites, and international and commercial ventures. Demand for...
-
Following the example shown in (a) below, indicate the effects of the listed transactions on the assets, liabilities, and stockholders equity of John Dallmus, certified public accountant, a...
-
What effect does the ordering of a search tree have on the efficiency of the search? What effect does it have on the quality of the results? How would order affect the way that depth-first search or...
-
For each of the accounts listed below, indicate whether the account is increased by a debit or a credit: Accounts Receivable Sales Revenue Equipment Common Stock Notes Payable Retained Earnings...
-
Smart Sports is also planning to launch a range of drinks products. The products have been developed by Hydration Labs Ltd and are designed to be sold as powders that dissolve easily in water. They...
-
Baucom Company accepted credit cards in payment for \(\$ 6,850\) of services performed during March 2011. The credit card company charged Baucom a 4 percent service fee. The credit card company paid...
-
Is one measure better than another?
-
Show that gj concave AHUCQ Abadie For nonnegative variables, we have the following corollary.
-
One of the reactions given in Fig. P11.76 is about 2000 times faster in pure water than it is in pure ethanol. Another is about 20,000 times faster in pure ethanol than it is in pure water. The rate...
-
Account for the following observations with a mechanism. (1) In 80% aqueous ethanol, compound A reacts to give compound B. Notice that trans-B is the only stereoisomer of this compound that is...
-
Draw a curved-arrow mechanism for each of the conversions shown in Fig. P11.77. Figure P11.77 S
-
Estimate the intrinsic value of the stock company ABC. Dividends were just paid at $8 per share and are expected to grow by 5%. You require 20% on this stock given its volatile characteristics. If...
-
Crane, Inc., a resort management company, is refurbishing one of its hotels at a cost of $6,794,207. Management expects that this will lead to additional cash flows of $1,560,000 for the next six...
-
Match each of the following transactions with the applicable internal control principle that is being violated

Study smarter with the SolutionInn App


