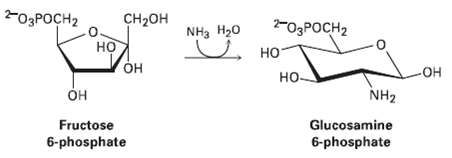
Glucosamine, one of the eight essential monosaccharide?s (Section 25.7), is biosynthesized as its 6-phosphate derivative from fructose
Question:
Glucosamine, one of the eight essential monosaccharide?s (Section 25.7), is biosynthesized as its 6-phosphate derivative from fructose 6-phosphate by reaction with ammonia. Propose a mechanism.
Transcribed Image Text:
-03POCH2 -O3POCH2 но- CH2он NH, H20 но он -он но- HO- Он NH2 Glucosamine 6-phosphate Fructose 6-phosphate
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 64% (17 reviews)
Nucleophilic acyl substitution and tautomerization lead to the formation of ...View the full answer

Answered By

Jaseena vafa
Hands-on experience at the graduation and post-graduation level education facility
Familiarity working at a College and University
department
Outstanding ability to handle syllabus at the
undergraduate and graduate curriculum
Remarkable ability to teach, inspire and develop young
people
Excellent written and oral communication skills
Strong organizational skills and proficiency with MS
office tools.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Galactose, one of the eight essential monosaccharide?s (Section 25.7), is biosynthesized from UDP-glucose by galactose 4-epimerase, where UDP = uridylyl diphosphate (a ribonucleotide diphosphate;...
-
L-Fucose, one of the eight essential monosaccharide?s, is biosynthesized from GDP-D-mannose by the following three-step reaction sequence, where (H)P?guano sine diphosphate (a ribonucleoside...
-
Mannose, one of the eight essential monosaccharide?s (Section 25.7), is biosynthesized as its 6-phosphate derivative from fructose 6-phosphate. No enzyme cofactor is required. Propose a mechanism....
-
Lungameni Enterprises (Pty) Ltd ("Lungameni Enterprises") manufactures product A, which it sells to local customers at a mark-up of 25%. Lungameni Enterprises currently absorbs its overhead costs on...
-
With another student, analyze the persuasive email message at Host Marriott (Figure 9.5 on page 232) by answering the following questions. 1. What techniques are used to capture the reader's...
-
When he was the top American administrator in Iraq, L. Paul Bremer III set a rule that upheld Iraqi law: anyone 25 years and older with a good reputation and character could own one firearm,...
-
Interglobal Company has two operating divisions in a semiautonomous organization structure. Division +, anster Picesanctter X, located in the United States, produces part XZ-1, which is an input to...
-
Twain Services offers leadership training for local companies. It employs three levels of seminar leaders, based on experience, education, and management level being targeted: guru, mentor, and...
-
Not yet answered Points out of 1.0 Faction Isabella Corp. can sell its product for $30 per unit. Estimated sales are 20,000 units. Fixed costs are estimated to be $300,000. What is the maximum...
-
The Chartered Financial Analyst (CFA) designation is the de facto professional certification for the financial industry. Employers encourage their prospective employees to complete the CFA exam....
-
Draw the structure of L-galactose, and then answer the following questions: (a) Which other aldohexose gives the same aldaric acid as L-galactose on oxidation with warm HNO3? (b) Is this other...
-
Gentiobiose, a rare disaccharide found in saffron and gentian, is a reducing sugar and forms only n-glucose on hydrolysis with aqueous acid. Reaction of Gentiobiose with iodomethane and Ag20 yields...
-
It is important that you use appropriate data gathering methods. Why is this the case?
-
Assume a Poisson distribution with =5.6. Find the following probabilities. a. X=1 b. X <1 c. X>1 d. X1 a. P(X=1)= (Round to four decimal places asneeded.) b. P(X <1)= (Round to four decimal places...
-
345879 The any reported the following January purchases and sales data for its only prauct. The company uses a perpetual inventory system. REQUIRED: Determine the cost assigned to ending inventory...
-
How do changing geopolitical landscapes, such as shifting alliances and emerging power centers, influence conflict resolution strategies, and what adjustments are necessary to address new global...
-
50 21 2. Determine the inclination and period of the satellite which produced the ground trace below. Show all calculations. Suteite 17 11-140-130-120-110 tonn an 20 6058 am 50 210 0 10 20 30 50 60...
-
This activity aims to provide practical experience in preparing tax forms related to business income and depreciation. It emphasizes the importance of accurate reporting and adherence to tax...
-
19. If the settle price for a T-bill futures contract is 96.75, what is the percent discount? Appendix
-
(a) Water flows through the nozzle of a garden hose. Find an expression for m in terms of line pressure P 1 , ambient pressure P 2 , inside hose diameter D 1 , and nozzle outlet diameter D 2 . Assume...
-
Answer the following questions in a complete sentence or two: a. How do you know if an acid is strong or weak? b. How do you calculate the pH of a strong acid solution? c. How do you calculate the pH...
-
Write an equation for the reaction of cyclohexene with m-chloroperbenzoic acid, C-O-O-H Cl
-
Write an equation for the acid-catalyzed reaction of cyclohexene oxide with water. Predict the stereochemistry of the product.
-
Write an equation for the reaction between ethylene oxide and a. CH3CH2CH2CH2MgCl followed by hydrolysis b. C6H5CH2MgBr followed by hydrolysis c. H2C=CHLi followed by hydrolysis d. CH3CC-Na+ followed...
-
Comparative financial statements for Weller Corporation, a merchandising company, for the year ending December 31 appear below. The company did not issue any new common stock during the year. A total...
-
Mrquered Mrquered
-
You plan to invest $10,00 today in an investment account earning 5% interest. You then plan to invest an additional $1,000 into this account each year for the next twenty years. How much money will...

Study smarter with the SolutionInn App


