Glucose exists in two forms having a 36 : 64 ratio at equilibrium. Draw a skeletal structure
Question:
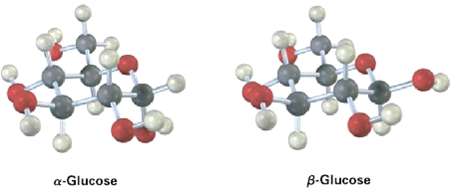
Glucose exists in two forms having a 36 : 64 ratio at equilibrium. Draw a skeletal structure of each, describe the difference between them, and tell which of the two you think is more stable (red = O).
Transcribed Image Text:
B-Glucose a-Glucose
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 57% (14 reviews)
HO HOCH HO OH H aOH HO HOCH HO OH OH Glucose Glucose The only d...View the full answer

Answered By

OTIENO OBADO
I have a vast experience in teaching, mentoring and tutoring. I handle student concerns diligently and my academic background is undeniably aesthetic
4.30+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Describe the difference between an account receivable and an account payable.
-
Describe the difference between project management and program management.
-
Describe the difference between inertia and brand loyalty.
-
Modify Index to make a program IndexByKeyword that takes a file name from the command line and makes an index from standard input using only the keywords in that file. Note: Using the same file for...
-
Calculate the annual interest and the semiannual interest payment for the following corporate bond issues with a face value of $1,000. Annual Interest Amount Semiannual Interest Payment Annual...
-
A school board has instituted a series of standardized tests in math, history, English, and science that all high school students must pass with a grade of 70 before they are allowed to graduate and...
-
What is the process for posting to a subsidiary ledger and its controlling account? AppendixLO1
-
Refer to Problem 16-1 and assume that the company had $3 million in assets at the end of 2008. However, now assume that the company pays no dividends. Under these assumptions, what additional funds...
-
Assume you are the partner in charge of the 201 9 audit of Becker Corporation, a private company. The audit report has not yet been prepared. In each independent situation following (1-8), indicate...
-
On January 1, 2014, Klinefelter Company purchased a building for $520,000. The building had an estimated life of 20 years and an estimated residual value of $20,000. The company has been depreciating...
-
A trisubstituted cyclohexane with three substituents? red, yellow, and blue?undergoes a ring-flip to its alternative chair conformation. Identify each substituent as axial or equatorial, and show the...
-
Draw the five cycloalkanes with the formula C5H10.
-
@VickiK wrote to JetBlue: I have booked a flt in July, CA-VT. Wondering about flying my wedding dress w/me. Is there a safe place to hang it on the plane? Prepare a response based on the following...
-
The University of Cincinnati Center for Business Analytics is an outreach center that collaborates with industry partners on applied research and continuing education in business analytics. One of...
-
For a data set of the pulse rates for a sample of adult females, the lowest pulse rate is 38 beats per minute, the mean of the listed pulse rates is x = 78.0 beats per minute, and their standard...
-
A student earned grades of A, C, B, A, and D. Those courses had these corresponding numbers of credit hours: 5, 3, 4, 3, and 2. The grading system assigns quality points to letter grades as follows:...
-
Ch 3: Forecasting: Tracking Signals, Mad, Exponential Smoothing, Control Charts Media Consultants (10 Pts). Media Consultants uses proven techniques to measure forecast accuracy and to determine when...
-
Question 2 What is the energy (in joules) of the photon absorbed by a hydrogen atom to cause a ground-state electron to move to the n = 3 energy level? Record your answer in scientific notation to 3...
-
1.6 A Select from the following list the most appropriate way of financing the build-up of stocks of Easter eggs in a chocolate business: a) venture capital b) overdraft c) issue of new share capital...
-
F.(3e* -2x 3 sin(2x)) is equal to 2 3 Cos 8. IT 3, t (4+@ 2 3, 1+o 1 4 Cos 4 4 1 3. 1 +4cos V7 (1+o 4 1 4 Cos 4 1+0 4-
-
Rank the following in order of increasing surface tension (at room temperature): (a) CH 3 OH; (b) HOCH 2 CH 2 OH; (c) CH 3 CH 2 OCH 2 CH 3 .
-
(a) What factor explains the observation that tertiary alcohols react with HX faster than secondary alcohols? (b) What factor explains the observation that methanol reacts with HX faster than a...
-
Assign oxidation states to each carbon of ethanol, acetaldehyde, and acetic acid. [Ol Ethanol Acetaldehyde Acetic acid
-
Provide a retrosynthetic analysis and synthesis for each of the following compounds. Permitted starting materials are phenylmagnesium bromide, oxirane, formaldehyde, and alcohols or esters of four...
-
Diplomatic Security Service provides Airport Transportation and Surveillance Service to Foreign Diplomats in Guyana. The company has two support departments - Information Systems and Equipment...
-
Q1: A disparity of bargaining power between the parties to a contract may result in unfair terms but a court is not likely to consider the contract unconscionable. Group of answer choices a. True b....
-
Life Tool Manufacturing has a system in place to recall products that prove to be dangerous at some time after manufacture and distribution. This represents which element of the due care theory?...

Study smarter with the SolutionInn App


