Grignard reagents undergo a general and very useful reaction with ketones. Methyl magnesium bromide, for example, reacts
Question:
Grignard reagents undergo a general and very useful reaction with ketones. Methyl magnesium bromide, for example, reacts with Cyclohexanone to yield a product with the formula C7H14O. What is the structure of this product if it has an IR absorption at 3400 cm?1?
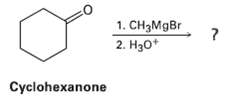
Transcribed Image Text:
1. CH3MgBr 2. H30* Cyclohexanone
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (16 reviews)
CH3 OH 1 CH3MgB...View the full answer

Answered By

Amir Farooq
I have done BSC from s.p college srinagar. After that i did my MBBS from achariya shri chander college of medical science sidhra jammu.currently i am an intern doctor at skims srinagar. I love to teach students across the globe. I was teaching students of 12 th class at my room from the last 5 years.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
What is the structure of ATCase?
-
What is the structure of the global beer industry?
-
What is the structure of the beer industry?
-
Night By Elie Wiesel The Holocaust - Why did the members of Sighets Jewish community refuse to believe their horrible situation? (Moshe the Beadle and Madame Schachter portending the horrors that...
-
How a firm behaves toward existing competitors is a major determinant of whether it will face entry by new competitors. Explain.
-
Do the energy-transfer diagrams in FIGURE Q21.9 represent possible refrigerators? If not, what is wrong? a. b. c. Hot reservoir 600 K 10 J 20 J |10 J 300 K Cold reservoir Hot reservoir 600 K 30 J 20...
-
Recently, Las Vegas has seen increased competition from Singapore and Macau (China) for customers in the casino resort industry. One measurement of success is the average length of stay by visitors....
-
On January 1, 2016, Bacco Company had a balance of $72,350 in its Delivery Equipment account. During 2016, Bacco purchased delivery equipment that cost $22,100. The balance in the Delivery Equipment...
-
If total liabilities decreased by $50,000 and stockholders' equity increased by $30,000 during a period of time, then total assets must change by what amount and direction during that same period
-
Leighton Industries needed steel pipe to build furnaces for a customer. Leighton sent Callier Steel an order for a certain quantity of A 106 Grade B steel. Callier confirmed the order and created a...
-
Methyl-2-pentanone and 3-methylpentanal are isomers. Explain how you could tell them apart, both by mass spectrometry and by infraredspectroscopy. H. 4-Methyl-2-pentanone 3-Methylpentanal
-
Ketones undergo a reduction when treated with sodium borohydride, NaBH 4 . What is the structure of the compound produced by reaction of 2-butanone with NaBH4 if it has an IR absorption at 3400 cm ?1...
-
Suppose that on January 18, 2022, a U.S. firm plans to purchase 3 million euros () worth of French bonds from a French FI in one months time. The French FI wants payment in euros. Thus, the U.S. firm...
-
You have recently taken over daycare center that was under substandard leadership. Currently, the staff is unmotivated, negative, and often absent from work. You notice that there is minimal...
-
Choose an organization from the industry of your choice to discuss, illustrate, and reflect deliberately on the following: Why is it important to distinguish between "group" and "team "? What kinds...
-
The focus of data governance programs, in some capacity, is enterprise-wide data quality standards and processes. If you were a manager focusing on master data: Would you likely meet enterprise-level...
-
1) Identify and explain each component of the ANOVA model. 2) How is the F ratio obtained? 3) What role does the F ratio play?
-
Make a BCG matrix table and place the following products from Apple: iPhone, iPad, iMac, iPod, Apple TV, Apple Watch, AirPod, and HomePod. Briefly describe why you have placed the products in the...
-
Is a quantitative study the correct type of research design to help Uber Eats better understand the future of the online food delivery industry?
-
a. What is the cost of borrowing if Amarjit borrows $28 500 and repays it over a four-year period? b. How many shares of each stock would he get if he used the $28 500 and invested equally in all...
-
Potassium perchlorate (KClO 4 ) has a lattice energy of -599 kJ/mol and a heat of hydration of -548 kJ/mol. Find the heat of solution for potassium perchlorate and determine the temperature change...
-
When cis-2-methylcyclohexanol reacts with the Lucas reagent, the major product is 1-chloro- 1-methylcyclohexane. Propose a mechanism to explain the formation of this product?
-
Write balanced equations for the three preceding reactions?
-
Suggest how you would convert trans-4-methylcyclohexanol to (a) Trans-1-chloro-4-methylcyclohexane? (b) Cis-1-chloro-4-methlcycloexane?
-
can anyone help find a news article discussing a corporations use of debt financing? Often times the financial news media will report when a company chooses to issue new bonds or take new loans.
-
a canadian investor puts money into an australian investment that offers an interest rate of 5% for six minths. The australian dollar appreciates by 6% over this period of six months. What is the...
-
Los siguientes datos corresponden a las operaciones de Turk Company el ao pasado: Ventas $ 900 000 Utilidad operativa neta $ 36 000 Margen de contribucin $ 150 000 Activos operativos promedio $ 180...

Study smarter with the SolutionInn App


