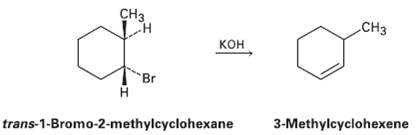
How can you explain the fact that franc-i -hromo-2-methylcyclohcxane yields the non-Zaitsev?s elimination product 3-methylcyclohexene on treatment
Question:
How can you explain the fact that franc-i -hromo-2-methylcyclohcxane yields the non-Zaitsev?s elimination product 3-methylcyclohexene on treatment with base?
Transcribed Image Text:
сHз Cнз кон Br trans-1-Bromo-2-methylcyclohexane 3-Methylcyclohexene
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 53% (15 reviews)
ring CH3 B4 flip Br CH3 Br cis H KOH trans diaxial E2 reac...View the full answer

Answered By

Somshukla Chakraborty
I have a teaching experience of more than 4 years by now in diverse subjects like History,Geography,Political Science,Sociology,Business Enterprise,Economics,Environmental Management etc.I teach students from classes 9-12 and undergraduate students.I boards I handle are IB,IGCSE, state boards,ICSE, CBSE.I am passionate about teaching.Full satisfaction of the students is my main goal.
I have completed my graduation and master's in history from Jadavpur University Kolkata,India in 2012 and I have completed my B.Ed from the same University in 2013. I have taught in a reputed school of Kolkata (subjects-History,Geography,Civics,Political Science) from 2014-2016.I worked as a guest lecturer of history in a college of Kolkata for 2 years teaching students of 1st ,2nd and 3rd year. I taught Ancient and Modern Indian history there.I have taught in another school in Mohali,Punjab teaching students from classes 9-12.Presently I am working as an online tutor with concept tutors,Bangalore,India(Carve Niche Pvt.Ltd.) for the last 1year and also have been appointed as an online history tutor by Course Hero(California,U.S) and Vidyalai.com(Chennai,India).
4.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
How would you explain the fact that Jupiter and Saturn have periods much greater than one year?
-
How do you explain the fact that some people make very high returns on stock markets?
-
How do you explain the fact that a single rose at the supermarket florist Is $1.49 every day of the year except the week before and during Valentine's Day, when it increases to $3.50?
-
Figlio (1999) found that legislators are more likely to mirror their constituents preferences during election years than in earlier years of their terms. This is particularly true for relatively...
-
Besides setting up a Pinterest account, what kinds of technical support would trainers need to provide if they want to use Pinterest to aid transfer of training?
-
Bannack Corp. is in the process of preparing its statement of cash flows for the year ended June 30, 2014. An income statement for the year and comparative balance sheets are as follows: For the Year...
-
List the different safety measure guidelines of a theme park in your country. LO.1
-
During 2021, its first year of operations, Pave Construction provides services on account of $160,000. By the end of 2021, cash collections on these accounts total $110,000. Pave estimates that 25%...
-
Bob Katz is purchasing a new Honda Pilot for $35,000. He is financing $32,000 with a six year, 4% loan with annual payments. Construct an amortization schedule, in the 2nd year row, corresponding to...
-
Glenrose Servicing began operations on June 1, 2020. The transactions for the first two months follow: Required 1. Create two tables like the one in Exhibit 1.15 for each of June and July using the...
-
In light of your answer to Problem 11.49, which alkene, F or Z, would you expect from an E2 reaction on the tosylate of (2R, 3R)-3-phenyl-2-butanol? Which alkene would result from E2 reaction on the...
-
Predict the product(s) of the following reaction, indicating stereochemistry wherenecessary: Br CH3 H20 Ethanol
-
An attorney's office operates with a single copier. At the beginning of a particular day the following jobs are waiting for processing. All jobs must be distributed to clients or in court by 9:00...
-
Gil and Ruth George have been friends of yours for many years. They have come to you for advice on their estate plan since they want a second opinion to make sure it is going to do what they hope....
-
The test statistic of z = 1.74 is obtained when testing the claim that p # 0.658. Identify the hypothesis test as being two-tailed, left-tailed, or right-tailed. Find the P-value. Using a...
-
Ann and Bob had their first date. Each either felt romantic chemistry (C) or no chemistry (NC) with the other person. Each person knows his/her own feeling but does not know the feeling of the other...
-
Find the following using countif, countifs, sumif, sumifs, averageif, and averageifs. Create all formulas and calculations directing in Excel. How many songs are sung by Moore? What is the average...
-
Total number of Ledgers, Groups, Entries etc. can be shown from o a. Tally Audit o b. Statistics o c. Accounts Information o d. Company Information
-
understand the basic principles underlying accounting systems and be familiar with the terminology;
-
What kind of rays are X-rays?
-
This reaction has an equilibrium constant of K p = 2.26 * 10 4 at 298 K. Calculate K p for each reaction and predict whether reactants or products will be favored at equilibrium. CO(g) + 2 H(g) =...
-
Cinnamaldehyde is used as a flavoring agent in cinnamon candies. Show how cinnamaldehyde is synthesized by a crossed aldol condensation followed by dehydration. cinnamaldehyde-C
-
Give the important resonance forms for the possible enolate ions of: (a) Acetone (b) Cyclopentanone (c) Pentane-2,4-dione (d) (e) (f)
-
Show how octane-2,7-dione might cyclize to a cycloheptenone. Explain why ring closure to the cycloheptenone is not favored.
-
During 2024, its first year of operations, Hollis Industries recorded sales of $11,900,000 and experienced returns of $760,000. Cost of goods sold totaled $7,140,000 (60% of sales). The company...
-
What is the value of a 15% coupon bond with 11% return? Is it a discount or a premium bond?
-
A manufacturer with a December 31 taxation year end sells new machinery for $50,000 on January 2, 2022. The cost of the machinery is $20,000. The terms of the sale require an initial payment of...

Study smarter with the SolutionInn App


